This is the monomer of a carbohydrate.
What is a monosaccharide?
Besides containing Carbon, this is a characteristic that all lipids share.
What is they are hydrophobic (do not mix with water)?
This is the monomer of a protein.
What is an amino acid?
This is the monomer of a nucleic acid.
What is a nucleotide?
Allows tiny creatures to walk on water
Surface Tension
These are the elements that make up carbohydrates.
What are carbon, hydrogen, and oxygen?
These are the four different types of lipids.
What are triglycerides, phospholipids, waxes, and steroids?
These are the elements that make up a protein.
What are carbon, hydrogen, oxygen, and nitrogen?
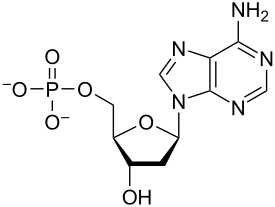 This macromolecule.
This macromolecule.
What is a nucleotide?
The two properties that allow water to move up plants Xylem walls.
What is cohesion and adhesion?
This is the ratio of elements that all carbons have.
What is 1:2:1?
Water will not dissolve lipids. However, will dissolve ionic and ____________ molecules
What is polar?
This speeds up a chemical reaction.
What is an enzyme?
These elements make up a nucleic acid.
What is carbon, hydrogen, oxygen, nitrogen, and phosphorous?
Water will dissolve ionic or ___________ molecules
what is polar?
 This type of macromolecule?
This type of macromolecule?
What is a carbohydrate?
:max_bytes(150000):strip_icc()/aldosterone-56a129883df78cf77267fc6b.jpg) This macromolecule.
This macromolecule.
What is a steroid (lipid)?
This is what happens when a protein [Example: Enzymes] unfolds losing structure.
What is denaturation?
These are the two types of nucleic acids.
What is RNA and DNA?
This type of bond between water molecules is important in living systems because
It makes water cohesive
It gives water a high specific heat capacity
It helps water to be a good solvent for polar molecules
What is hydrogen bonding?
The type of reaction that breaks down a polysaccharide into a disaccharide
What is hydrolysis?
What is long term energy?
When a dipeptide is formed from two amino acids, this type of reaction takes place.
This is the function of DNA and RNA.
What is DNA codes for genetic information and RNA codes for protein production?
You are overheating and sweat to return to a normal temperature. The sweat uses energy from your body to change forms causing you to feel cool.
What is evaporative cooling?