How dangerous a chemical is to your health, an organ, another organism, or to the environment
What is toxicity?
When the appearance of a substance changes, but the individual modules stay the same. NO new substance is made.
What is a physical change?
The vertical columns and horizontal rows on a periodic table
What are groups/families and periods?
A pure substance that cannot be separated into a simpler substance
What is an element?
On the far right side of the periodic table
Where are non-metals located on the periodic table?
Any substance that has mass and takes up space
What is matter?
The feel, appearance, or consistency of a surface or substance
What is texture?
When a substance loses its original identity and forms a new substance with another substance
What is a chemical change?
The number of protons in an element
What is an atomic number (the top number)?
When elements chemically combine they become a compound and form a new substance
What is a compound?
On the far left side of the periodic table
Where are metals located on the periodic table?
How much space an object takes up (height, width, and depth)
What is volume?
Solid to liquid to gas
What is state of matter?
Name the change that change of color shows
What is an example of chemical change?
The microscopic mass of an element
What is atomic mass (the bottom number)?
To figure out the number of elements in a compound (ex: H2O = 2)
Why do we look at capital letters?
On the staircase on the right side of the periodic table
Where are metalloids located on the periodic table?
Mass ÷ Volume
What is the formula for density?
Ability to be drawn out into thin wires
What is ductility?
Many can be undone
What is a physical change?
18 (columns) 7 (rows)
How many groups and periods are there?
Name what this statement means
NaHCO3= 4
How many elements are in NaHCO3?
Name the metalloid that has the atomic number of 33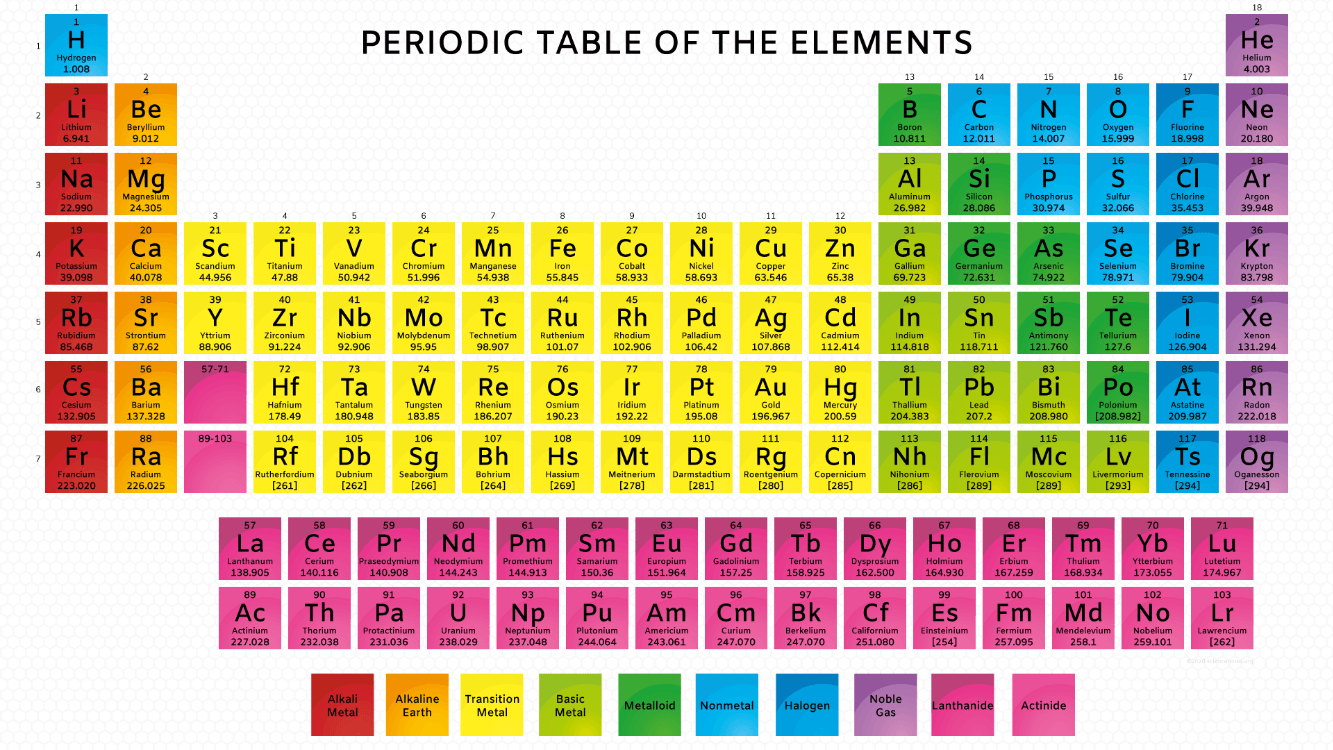
What is arsenic?
Water displacement (what is it for?)
How do you find the density/volume of an irregular object?
Elements that have properties such as high luster, high conductivity, brittleness, and softness
What are metalloids?
Name the change that mixing a smoothie together shows
What is an example of a physical change?
Name the element with the atomic mass of 197.0 (ROUNDED)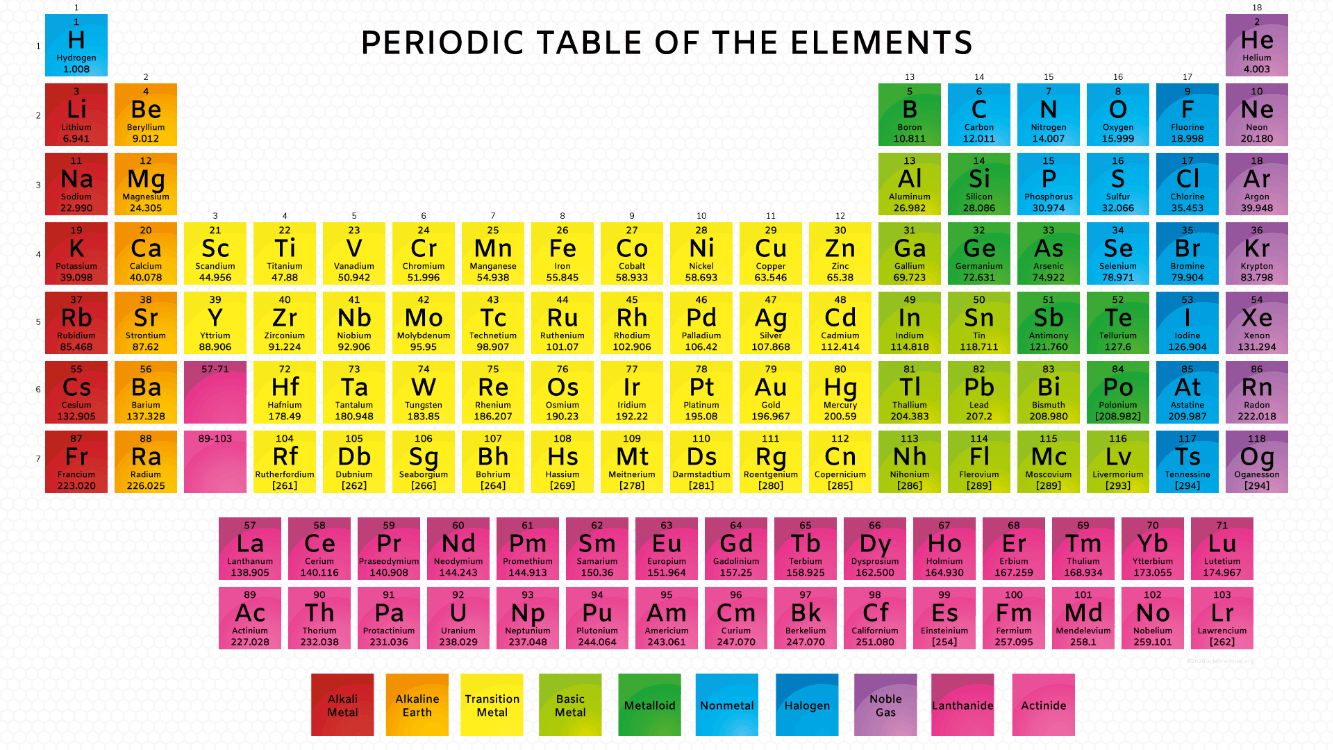
What is the atomic mass of gold?
Name the number of elements NaCl has
What is the number of elements NaCl has? (2)
Name the type of metal (hint: metal, or non-metals, or metalloids) that could be a solid, liquid, or a gas in room temperature.
What are non-metals?
127 mass, 2 volume = ...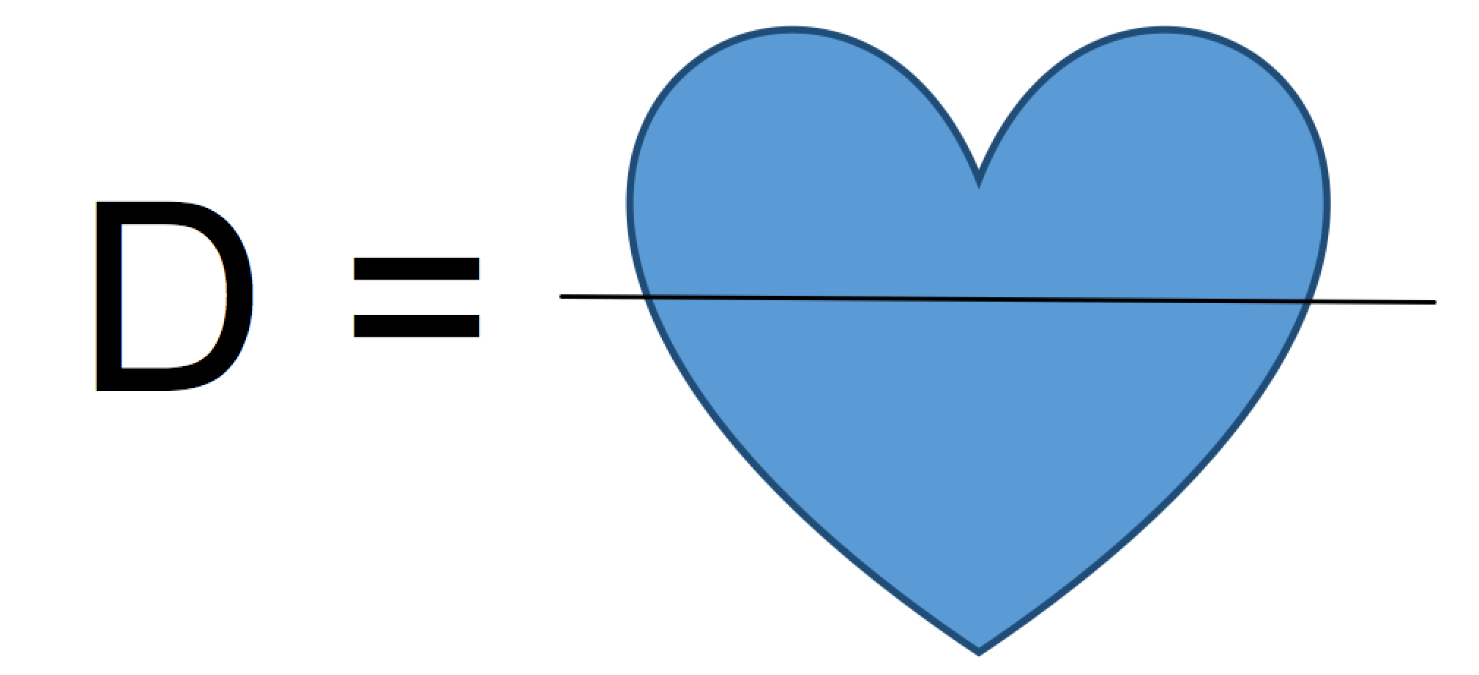
What is the density of this object? (63.5)