Which substance should a student apply to the skin if he or she gets splashed with an acid?
A. water
B. vinegar
C. salt
D. formaldehyde
A. water
What is mass, and what units are they measured in?
Mass is the amount of matter (stuff) that a substance contains
Measured in grams typically.
Describe density and give the units it is measured in.
How tightly packed molecules are. Grams per liter
Describe volume and the give the units.
Volume is the amount of space something takes up (ml)
Double or nothing: give the units for measuring volume of a solid.
Method of using initial and final position to find movement such as volume of an object:
A. Displacement
B. meters
C. Difference
D. Division
A. Displacement
In a laboratory setting, a student is heating a solution of salt water in a flask. The student notices that the bottom of the flask has a small crack. Which of these would be the safest procedure to follow?
F. keep heating the solution because the crack is small
G. stop heating the solution and try to tape the crack
H. stop heating the solution and notify the teacher
J. keep heating the solution but lower the temperature
H. stop heating the solution and notify the teacher
What lab instrument did we use to measure mass during our lab experiment?
Triple beam balance
Which is more dense: olive or vegetable oil, and how do you know?

Vegetable oil, because it sinks under the olive.
Double or nothing: Name one physical property of olive oil makes it more dense than vegetable oil?.
What is the volume displacement of the hammer?
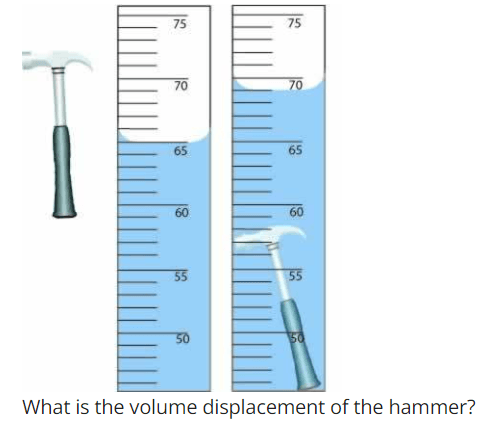
What is 4 mL
Double or nothing: What is the name of this instrument
Water has a specific density of 1 g/ml3.
If an object has a density of .6 g/mL and you put it into water, will it sink or float? Explain.
float bc it's less dense
Which of these is the best way to dispose of a potentially dangerous compound?
A. pour it down the drain
B. put it into a garbage can
C. recycle the compound for a future lab activity
D. place it in a hazardous material waste container
D. place it in a hazardous material waste container
Density = 24 g/mL
Volume = 6 mL
What is Mass = 144 g?
Mass = 270 g
Volume = 9 mL
What is Density = 30 g/mL
Density = 8 g/mL
Mass = 92 g
What is Volume = 11.5 mL
A sample of which element could have a volume of 9.37 cubic centimeters (cm3) and a mass of 44.88 grams (g)? (Use the chart to the below)
Selenium
Which of the following is an example of irresponsible behavior in the science lab?
F. testing new hypotheses with the teacher’s supervision
G. covering liquids before heating them
H. sweeping up broken glass
J. observing chemical reactions
G. covering liquids before heating them
What is the difference and similarity between weight and mass?
Similar: Both used to measure how heavy something is.
Differences: Mass doesn't change; however, weight is a force determined by gravity, and it changes depending on where you are.
Mass = 454 g
Volume = 227 mL
What is Density = 2 g/mL?
Density = 2.83 g/mL
Mass = 96.38 g
What is Volume = 34.05 mL
Name one substance found on Earth which is more dense in solid than in liquid form.
What is water?
Name three readily handy kitchen supplies that you can use if a fire breaks out in your kitchen.
Baking soda, salt, pot lid
Double or nothing: explain why these are effective.
As an object's mass increases, its gravitational pull will ____________.
A. decrease B. increase C. stay the same D.get weaker
B. increase
Density and volume are: (Choose one and explain)
A. directly proportional
B. inversely proportional
C. constant value
D. None of the option
B. inversely proportional because as volume increases, density decreases and visa-versa.

If a container full of air molecules were opened, which of the following properties would change?
A. Volume
B. Density
C. Mass
A. Volume
B. Density
Double or nothing: explain why.
If the box has a mass of 72 grams, what is its density?
A. 96 g/cm3
B. 48 g/cm3
C. 24 cm3
D. 3 g/cm3
D. 3 g/cm3
