Describe the motions of particles in a solid.
Describe the motion of particles in a liquid.
How are elements and compounds similar?
A. They are both on the periodic table.
B. They are both pure substances
B. They are both pure substances.
Density is a physical property. What is it's formula?
D= m/V
What happens to iron exposed to air?
Rust
What is a property of ONLY solids?
A. Particles do not move
B. Definite shape
B. Definite shape
A child is upset because his Rita's Ice is melting. He thinks he now has less dessert. Which of the following states why the child is incorrect.
a. He actually has more, not less, ice cream.
b. No mass is lost during a change of state.
B. No mass is lost during a change of state.
What is anthing that has mass and takes up space?
Matter
Jessica is measuring density. She put a ball on a balance to find mass. What else does she need?
a. scale
b. water
c. graduated cylinder
What is a graduated cylinder
One chemical property that can be measured in a substance is its reactivity with water. What is another chemical property?
a. density
b. malleability
c. flammability
c. flammability
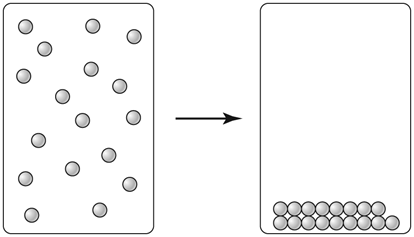
Based on what you know about matter, explain why the diagram shows a physical change rather than a chemical change.
A. The particles get smaller
B. The composition of the matter stays the same.
C. The volume of the matter decreases.
Both B and C are correct statements. However, in a physical change, composition of matter always stays the same.
What happens to the temperature of a sample of matter as it changes from a liquid to a gas?
A. The temperature increases
B. Temperature decreases.
C. Temperature remains the same.
C. Temperature remains the same.
Matter is made up of particles. Which of the following statements is true about these particles?
A. The particles that make up solids do not move.
B. The particles that make up all matter are constantly in motion.
B. The particles that make up all matter are constantly in motion.
Jeudy partially filled a graduated cylinder with water. He then drops a rock in the water. Which property of matter is Jeudy measuring?
A. Volume
B. Weight
C. Length
Which is a sign of a chemical change?
a. Change in mass.
b. Change in density.
c. Change in color.
c. Change in color.
On a hot day, a puddle dries up. Which statement describing this event is true?
A. The water in the puddle changes into particles of oxygen in the air.
B. The water in the puddle is destroyed.
C. The water in the puddle evaporates into water vapor.
C. The water in the puddle evaporates into water vapor.
A water molecule is made up of one oxygen atom and two hydrogen atoms. Why is water considered a pure substance?
A. Each water molecule is identical.
B. Water can be combined with other substances by physical means.
A. Each water molecule is identical.
What is the volume of a cube that is 10cm x 2cm x 10cm?
200 cm3
Which of the following is a chemical property of water in the beakers?
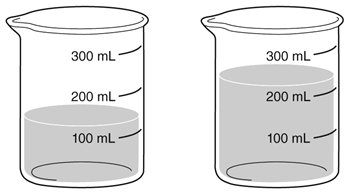
A. The combined volume of the water is about 400mL.
B. Water freezes at O Celsius.
C. Water reacts with some metals.
C. Water reacts with some metals.
Solid dry ice changes directly into carbon dioxide gas. This change of state is known as. Explain.
Sublimation.
Particles on the surface of the solid gain enough energy to break free into the gas phase.
Describe the motion of particles in the gas phase.
Particles move fast and freely filling their container.
What is the density of a rock? A correct answer must have correct units.
mass= 50g
volume=10mL
5 g/mL
You put a rock in a graduated cylinder to find the volume. The beginning volume is 25mL and the ending volume is 67mL. What is the volume of the rock?
42mL
How does temperature affect chemical change?
An increase in temperature increases the rate of a chemical change.