This is the building blocks of all matter
Atoms
What is the atomic number for Fe
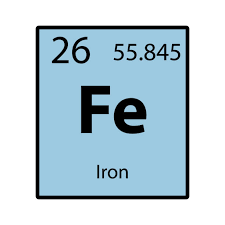
26
The starting substances of a chemical reaction are called
Reactants
How many atoms of Sodium are in this chemical formula NaCl
All of the atom's weight can be found here
Nucleus
This subatomic particle can be found in the electron cloud
electrons
Atomic number is the same as the number of
Protons
The subscript tells you what
The number of atoms
How many atoms of Hydrogen
2H + HCL
3
This is the simplest element on the periodic table
Hydrogen
This subatomic particle has a positive charge
protons
Atomic numbers increase in increments of _____ on the periodic table
1
What is the coefficient
4NaOH2
4
What is the total number of atoms 3CO
6
Which equation is the reactant 2HCl -----> H + 2Cl
2HCl
These subatomic particles are located in the nucleus
protons and neutrons
All elements on the periodic table are considered this
a pure substance
The coefficient tells you _____
number of molecules
When a chemical equation has parenthesis with a subscript next to you should know to do this?
Multiply
What is the most abundant element in Earth's atmosphere?
Nitrogen
What is the charge of the nucleus
positive
How many neutrons does Fe have
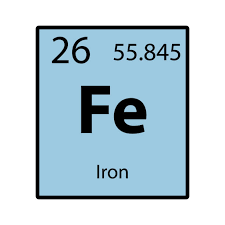
30
NaOH+ HCl is called a
Chemical equation
Matter cannot be created nor destroyed is known as this
The law of conservation of matter
In this chemical formula 4NaCl what does the 4 tell you?
How many molecules of NaCl are present or that you have 4 molecules of NaCl