If a student wanted to determine how much matter was in an object, which tool would be the best to use? Select the best answer:
Pan Balance
Ruler
Graduated Cylinder or Measuring Cup
Thermometer
Pan Balance
Pick something that is NOT matter. Explain why it is not matter.
Energy, force, feelings, thoughts, etc
because matter has to be something that takes up space and has mass.
Which statement is true? Select the best answer
1. Solutions are not mixtures
2. When a substance dissolves in a solution, the substance is no longer there.
3. When a substance dissolves in a solution, the particles might float on the top or sink to the bottom.
4. Using physical properties is a way to separate mixtures and solutions.
4. Using physical properties is a way to separate mixtures and solutions.
What is an advantage of this model:
1. It shows how the atoms in a liquid are arranged to make a defined shape.
2. It shows how the atoms in a gas have a defined volume (take up a set amount of space).
3. It shows how the atoms in a solid can move easily around one another.
4. It shows how the atoms in a gas can fill a space.
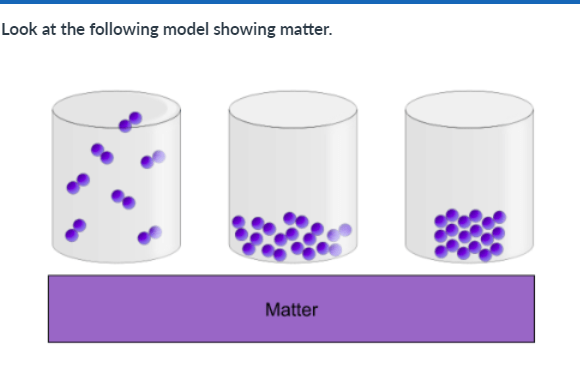
4. It shows how the atoms in a gas can fill a space.
A student wanted to conduct an investigation with a solid that had a mass of 50 grams. She wrote her observations in this data chart.
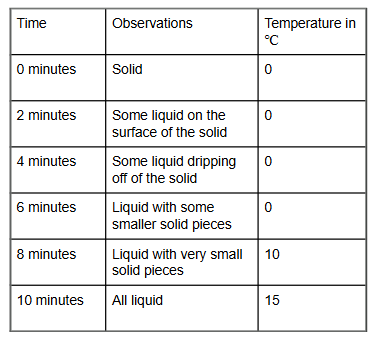
What other data is needed to show that mass was conserved when the matter changed phases?
1. She needs to measure the mass of the liquid and the solid at 4 minutes
2. She needs to measure the mass of the liquid at 10 minutes
3. she needs to measure how long it takes to change the liquid into a gas.
4. She needs to look to see if the solid and the liquid look like the same amount.
2. She needs to measure the mass of the liquid at 10 minutes
A student conducted an investigation combining two substances, water and sugar. Complete this data table to show that mass was conserved when the matter was mixed.
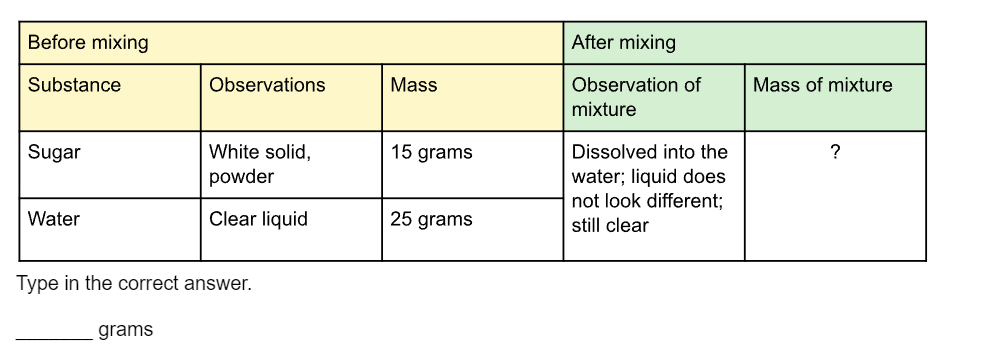
40 grams
Which statement is true about atoms? Select ALL the correct answers:
1. Atoms of different substances have different properties
2. Atoms are small pieces of matter that you cannot see.
3. Atoms are not affected by heat.
4. Atoms are found in all liquids, solids, and gases.
1, 2, 4 are all TRUE
(3 is false- Atoms ARE affected by heat)
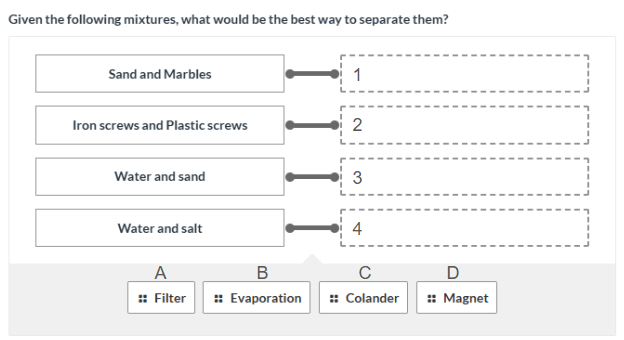
Sand and Marbles - Colander (1C)
Iron screws and plastic screws - magnet (2D)
Water and sand - Filter (3A)
Water and salt - Evaporation (4B)
A limitation of this model is that it does not show:
1. How the atoms in matter might look in various phases
2. The actual size of atoms
3. How liquids, solids, and gases differ in how they fill the container
4. How the atoms in a liquid may move more easily to flow past one another.
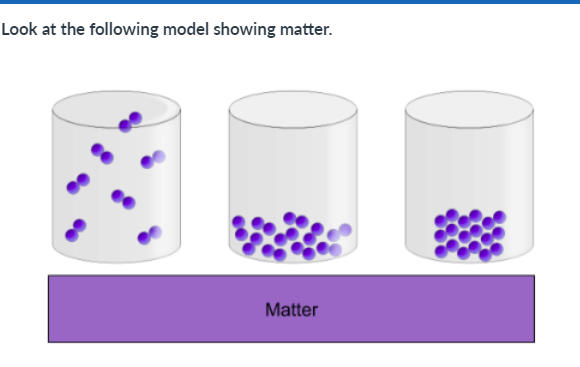
A limitation of this model is that it does not show:
2. The actual size of atoms
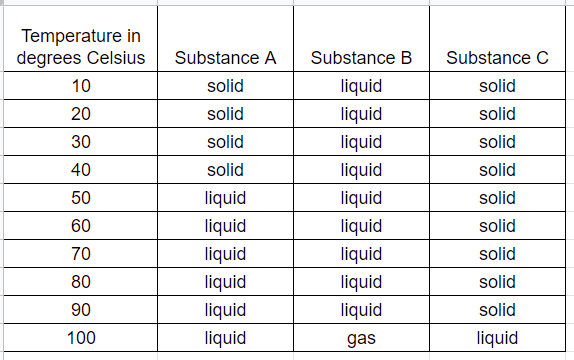
What was the student's testable question?
1. What is the effect of different substances on temperature?
2. What is the effect of whether something is a liquid or a solid?
3. What is the effect of temperature on phase changes in different substances?
4. What is the effect of different substances on solids, liquids, and gases?
3. What is the effect of temperature on phase changes in different substances?
A student conducted an investigation where liquid was transformed to a gas. Look at this data table and give a possible explanation as to why it appears that matter was not conserved.
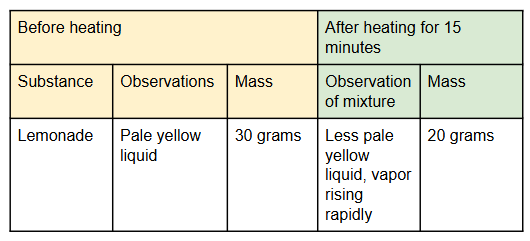
When liquid was heated, the gas began to evaporate; the mass was less than it was before heating because the mass of the gas could not be measured.
How does energy transform matter?
Use science words in your answer.
Energy transforms matter by causing it to change phase. Heat energy causes matter to go from a solid to a liquid to a gas. Removing heat energy causes the matter to cool, returning it to a liquid or a solid.
What would you do to separate salt, water, and small pieces of metal which were mixed together?
Use a magnet to separate the metal and then evaporate the water (using heat energy) to a gas leaving the salt behind.
Which set of models best demonstrates matter with the same volume and different mass? Select the best answer:
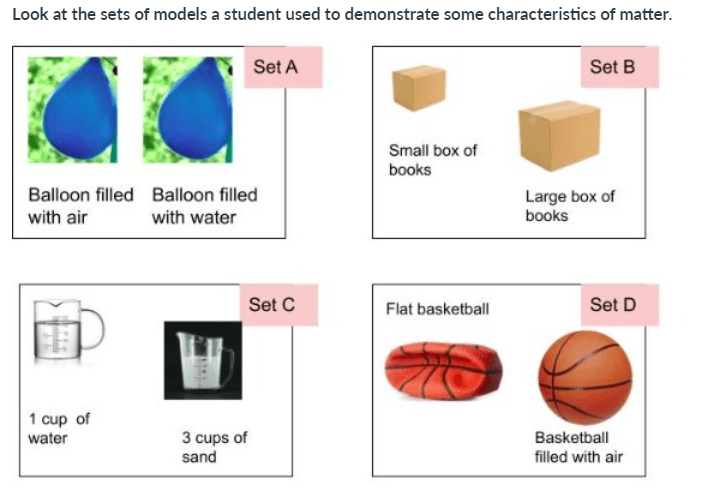
Set A
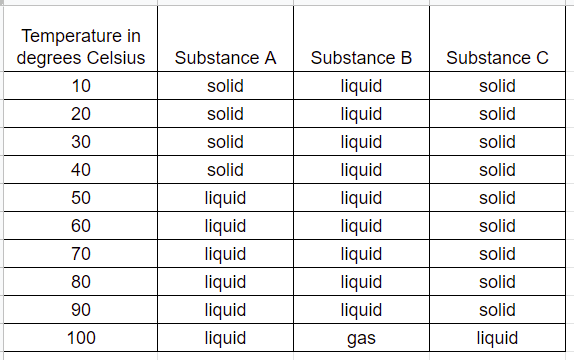
In this investigation, what would you expect to happen if heat energy was removed? Select the best answer.
1. The temperature would increase and gas might change phase to liquids.
2. Solids might change phase to liquids.
3. The mass would increase.
4. The temperature would decrease and the gas would change phase to a liquid.
4. The temperature would decrease and the gas would change phase to a liquid.
Tyler is making a ________. He starts with a jar full of 200 grams of water. He adds tea that has a mass of 50 grams and sugar with a mass of 20 grams. The tea and sugar dissolve in the hot water. What did he create and how many grams of the new substance should be in the jar at the end?
A. 150 grams
B. 270 grams
C. 200 grams
D. 130 grams
He created a solution and the mass is B. 270 grams
Which of the following describes a liquid:
A. Has a defined shape but no defined volume
B. Has a defined shape and defined volume
C. Has no defined shape but does have defined volume
D. Has no defined shape and no defined volume
C. Has no defined shape but does have defined volume
These objects are made from the same metal. What is a physical property they all have in common? What is a physical property that is different?
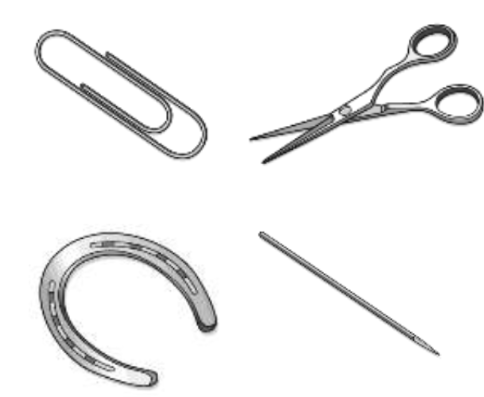
Different mass and volume (texture, size, shape)
Which of the following models demonstrates a mixture that is a solution and which one demonstrates a mixture that is not a solution?
*Bonus 200 points if you can identify an advantage of the model and a limitation of the model*
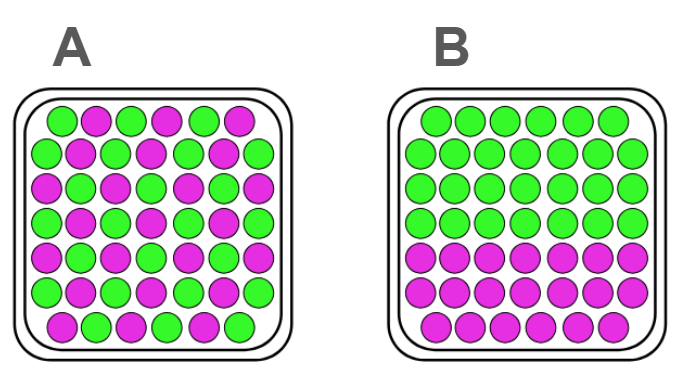
A - Mixture that IS a solution because the particles are evenly distributed showing something has been dissolved.
B- Mixture that is NOT a solution because the particles have not dissolved.
Identify the variables in the following experiment:
A student wanted to find out if crushed ice melts faster in water than ice cubes.
Independent variable:
Dependent variable:
Constants:
IV: Type of Ice (crushed or cubes)
DV: Time it takes for the ice cubes to melt (speed)
Constants: Temperature of water, amount of water, mass of ice, environment
Which graph accurately shows the amount of mass over time when ice is melted into a liquid and then frozen back into ice:
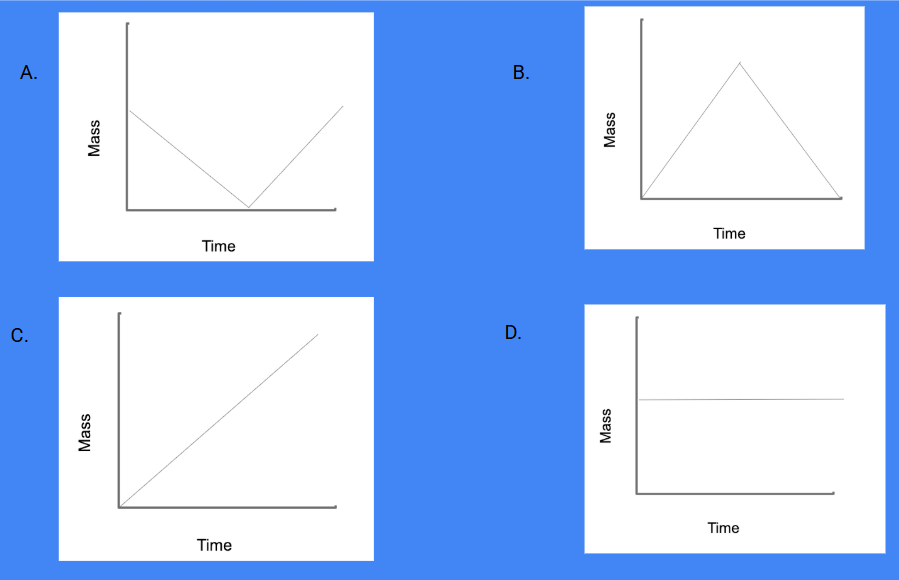
D. The amount of Mass and Matter is conserved and does not change even when the matter transforms to a different phase.
Which of the following describes a Gas:
A. Has a defined shape but no defined volume
B. Has a defined shape and defined volume
C. Has no defined shape but does have defined volume
D. Has no defined shape and no defined volume
D. Has no defined shape and no defined volume
What physical property is the same? What physical properties is different?

Same - Volume, color cup
Different - Mass
A limitation of the model below is that it does NOT show:
A. How fast the atoms are moving
B. That atoms in a gas fill the container
C. That atoms in a solid are tightly packed
D. That atoms in a liquid take the shape of the container
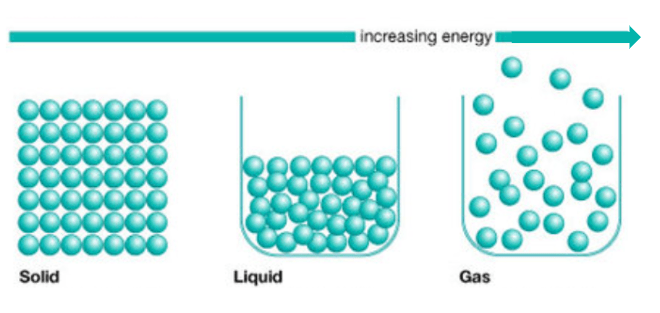
A. How fast the atoms are moving
Identify the testable question in the following experiment:
A student wanted to find out if crushed ice melts faster in water than ice cubes.
Testable Question:
Testable Question: What is the effect of ice shape on time it takes for the ice to melt?