What property describes how elements are dangerous because they are unstable and fall apart easily to more stable elements?

Radioactive
Give one chemical change example and one physical change example
Answers may vary
Physical changes would simply change the look of the object without changing the chemical formulas of the ingredients
Chemical changes would change the look of the object BY changing the chemical formulas of the ingredients
Endothermic reactions ______.
A) release more energy than they absorb
B) absorb more energy than they release
C) release and absorb exactly the same amount of energy
D) can either release more energy than they a
B) absorb more energy than they release
1) What two variables can you change to INCREASE the KINETIC ENERGY of an object?
2) What two variables can you change to INCREASE the POTENTIAL ENERGY of an object?
1) Add more mass and add more speed
2) Add more mass and add more height
Determine if the following is a Physical or Chemical Property of each substance:
#1 - Ability of Copper to conduct electricity easily
#2 - Ability of Copper to not dissolve in water
#3 - Ability of Copper to rust over time
#1 - Physical (the Chemical formula of the substance (Cu) stays the same, even when electricity runs through it)
#2 - Physical (the chemical formula of the substance stays the same, no chemical bonds are broken)
#3 - Chemical (the chemical formula of the substance changes, from Cu --> CuO or from copper to copper oxide)
What is the BEST way to tell a chemical change has occurred in ALL chemical reactions?
A new (or different) substance is formed.
The new substance would have different properties than the initial substance.
Na (sodium: explosive metal) + Cl (Chlorine: poisonous gas) = NaCl (non explosive, non poisonous table salt)
Liquid water sits in a cup. The cup is then put into a freezer, in which the water eventually freezes to ice.

Was this an exothermic process or an endothermic process?
Exothermic (heat was released OUT of the water to become cold enough to freeze).
How many different elements are present in this compound formula?
How many atoms total are present?
C6H7NaO6
Total Elements = 4: C H Na O
Total Atoms = 20: 6 + 7 + 1 + 6
What property would describe the image:
Aluminum foil is______________ because it can easily be bent into shapes and flattened

Malleable or Malleability
Is the following a physical change or a chemical change? Explain your answer. (2 minutes)

Trick question - BOTH (depends on where you look)
Some substances only change state making them physical changes (solid chocolate into liquid chocolate, but still chocolate)
Some substances change into something new (cookie dough changes chemicals to form new colors and smells)
Describe 1 exothermic process and 1 endothermic process from the following image:

Exothermic - Fire is releasing heat OR the pan releases heat into the ice.
Endothermic - Metal pan taking in the fire's heat OR Ice is melting (taking in the pan's heat)
Label each letter below AND tell me what type of wave this is
A - Wavelength
B - Crest
C- Resting Point
D- Amplitude
E- Trough
Type - Transverse Wave
Give 2 physical and 2 chemical properties of the object in the image:

Answers may vary
Physical - Brown, solid, smooth, heavy, etc.
Chemical - Flammable, does not react with water, etc
Decide if each example is a physical or chemical change:
#1 - Liquid CO2 evaporating to form gaseous CO2
#2 - Sugar dissolving in alcohol
#3 - Soft Marshmallows turning hard while sitting out on a counter
#4 - Food coloring added to water to make it a different color
#1 - Physical (still CO2)
#2 - Physical (still sugar and alcohol, just mixed)
#3 - Chemical (the outer coating is a new substance that wasn't there initially, the sugar reacts with oxygen to make this coating)
#4 - Physical (still water and food coloring, just mixed)
Forming a chemical bond _____.
A) absorbs energy from the surroundings
B) releases energy into the surroundings
C) creates energy that did not previously exist
D) uses up energy that then no longer exists
B) releases energy into the surroundings
Put all labels in order from oldest to youngest
-(J, V, M, R, T are rock layers)
-(GF is a fault, CU is erosion, also called an unconformity) 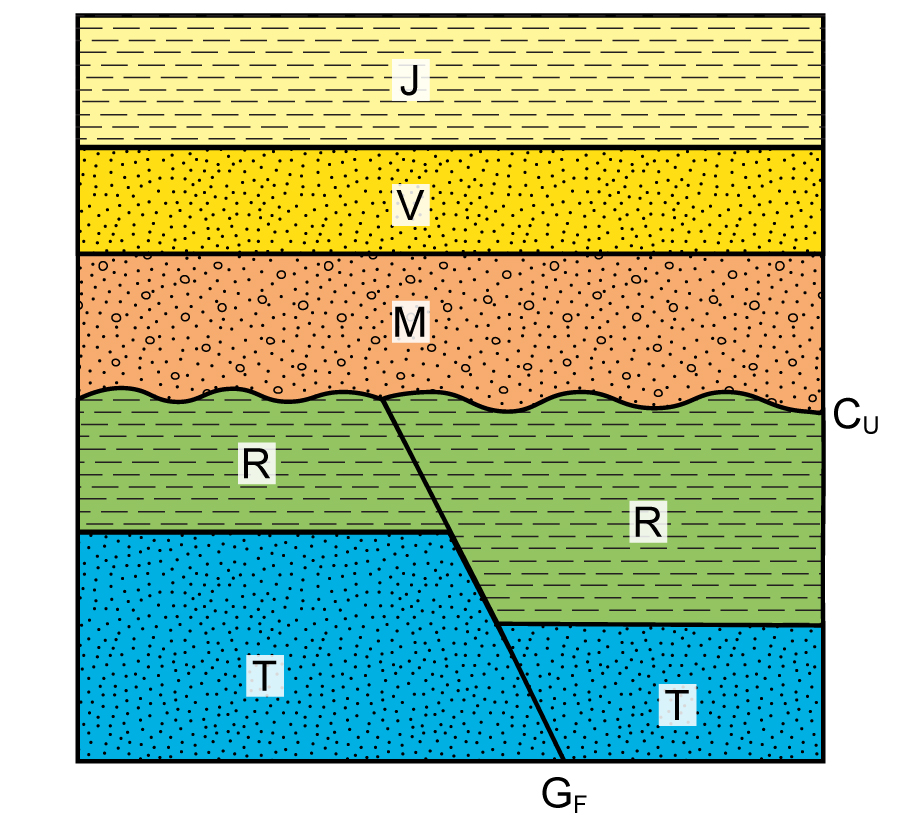
Oldest > T > R > GF > CU > M > V > J > Youngest