There are two types of pure substances, name them.
Elements and Compounds
What was the "Trick" Mr. Staiger taught you to remember how to convert between different metric prefixes?
King Henry Danced Merrily(base unit) Down Central Main
What does the law of conservation of mass state?
Matter/mass cannot be created nor destroyed, it must be conserved.
What is the measured value in the following problem:
A reaction between one mole of sodium and one mole of chloride should produce 42 grams of sodium chloride. In your experiment, the measured mass is 32.73 grams. Calculate the percent error of your experiment.
32.73 g
How many sig figs in the following measurement?
2,000 cm3
1
Two solid samples each contain sulfur, oxygen, and sodium, only. These samples have the same color, melting point, density, and reaction with an aqueous barium chloride solution. It can be concluded that the two samples are the same:
a. compound
b. element
c. mixture
d. solution
A! Same physical and chemical properties can only mean that it is the same substance!
Convert 20.0 cm to m
0.200 m
Filtration
Convert the following to scientific notation:
3000 cm
3.0 x 103 cm
How many sig figs in the following measurement?
0.0100 g
3
The classification for NaCl (aq) is...
homogeneous mixture/solution
Convert 2 kg to g
2000 g
This technique separates substances based on their differences in boiling point.
Convert to standard notation:
4.21 x 102 m
421 m
What are the rules when adding/subtracting with sig figs?
The answer should be rounded to the same amount of decimal places as the given number with the least number of decimal places.
What does aqueous mean?
Dissolved in water
How many eggs are there in 2.75 dozen?
Mixtures can be separated physically, while compounds can be separated chemically...how do you separate an element?
You can't!
What is the number before the "x 10n" called?
The mantissa
How many sig figs should be in the answer?
0.100 x 1001 = ?
3
Dissolving copper (II) chloride in water is classified as a physical or chemical change?
Physical!
How many seconds are there in 4.5 hours? Do some dimensional analysis!
16,200 seconds
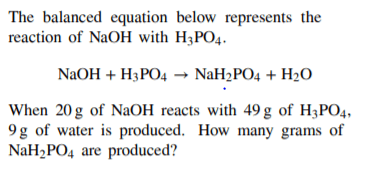
60 g
Where can you find the percent error equation?
Table T
What is the answer to the following (to the correct number of sig figs)?
1.01 g + 2.2 g = ?
3.2 g (MUST SAY GRAMS AS THE UNITS)