anything that has mass and takes up space
matter
Does mass or weight change depending on a planet's gravitational strength?
Only weight changes, mass stays the same.
___ a change of matter from one form to another without a change in chemical properties.
physical change
This property of water allows water striders and other insects to walk along its surface
What is surface tension?
What is pressure?
Sodium chloride is considered a _____ __________ because its composition is the same whether you're looking at a single grain of salt or a boulder of salt taken from a salt mine.
pure substance
What is the volume of the jade sample?
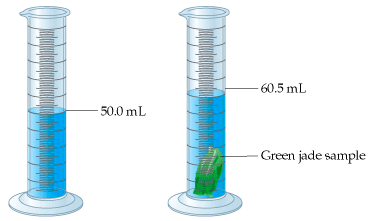
10.5 mL
___ a change that occurs when one or more substances change into entirely new substances with different properties.
chemical change
The total kinetic and potential energy of all the particle in an object or substance
What is thermal energy?
Which two laws describe the directly proportional relationship between volume and temperature and pressure and volume?
Charles's Law and Boyle's Law
______ , _________ , __________ , and __________ can all be classified at pure substances, because their composition is the same regardless of the amount.
Atom, elements, molecules, and compounds
Which solids from the table will float on water?
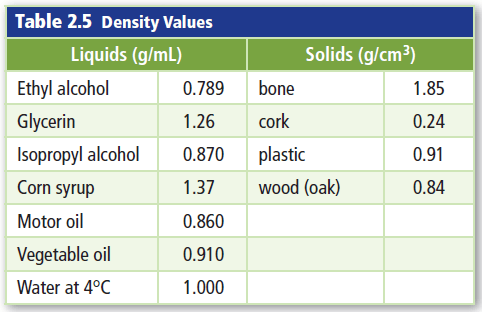
cork, plastic, and wood (oak)
Matter is not created or destroyed in any chemical or physical change
What is the law of conservation of mass?
How do liquids with a high viscosity differ from liquids with a low viscosity?
Liquids with high viscosity move more slowly and are thicker than liquids with a low viscosity
Which law relates the volume of a gas to it temperature at constant pressure?
Charles's law
Suppose you find a website that describes the rusting of a nail as a physical change of iron. Would that be accurate? Explain.
No, rust forms when a chemical changes takes place between iron and oxygen forming a new substance, iron oxide, which has different chemical properties than iron or oxygen.
Find the density of a 2 cm x 2 cm x 2 cm cube with a mass of 56 g.
7 g/cm^3
A northern right whale migrates from warm waters off Florida to cooler waters off Nova Scotia, Canada. In which area do you think more thermal energy would move from the whale to its environment? Explain.
Off Canada; thermal energy probably flows from the whale to its environment at all times, but more so when its environment gets colder.
What is sublimation?
When the temperature of a gas increases, and volume is fixed, what happens to the gas pressure? Why?
When temperature increases at constant volume, pressure increases. The particles become more energetic when temperature increases, so they exert more force on the container's walls.
What kind of mixture is mayonnaise?
homogeneous
The density of pine is generally about 0.5 g/cm3 . What is the mass of a 700 cm3 piece of pine? Hint: density = mass/volume
350 grams or 0.350 kilograms
A friend notices that a nail that was left outside for a few months seems larger and heavier than it was before. He says it disproves the law of conservation of mass. Explain why he is wrong.
Some of the added mass on the nail is from oxygen gas, which was in the system but was not accounted for because it's invisible.
How do the particles in a liquid create surface tension?
The particle below the surface attract the ones above, creating a "skin" on the top of the liquid.
Boyle's law