Paper
solid
Atom = 1
Molecule = 0
Element = 1
Compound = 0
Kira had a wide graduated cylinder that was filled up to the 100 mL line. She poured all of the water into a skinny but taller container. How much water does she have now?

Same amount 100ml
What is a covalent bond?
When two atoms share electrons
Light is matter, true or false? Explain!
False, it is energy.
Popsicle
solid
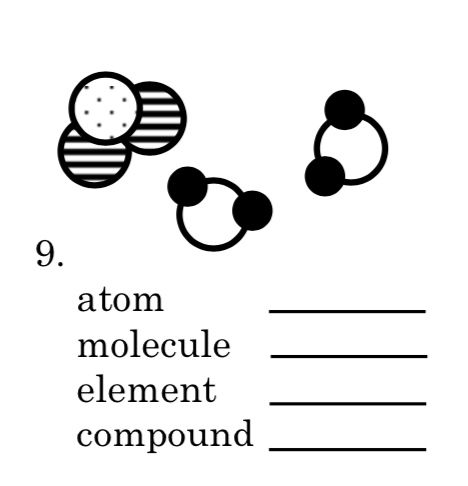
Atom = 4
Molecule = 2
Element = 2
Compound = 0
When the elevation (height) increases, the air pressure _________.
decreases
What is an ionic bond? Give one example
When atoms gain / lose electrons, forming a bond. Salt (NaCl)
Which states of matter do NOT have a definite shape?
liquid and gas
Oxygen
gas
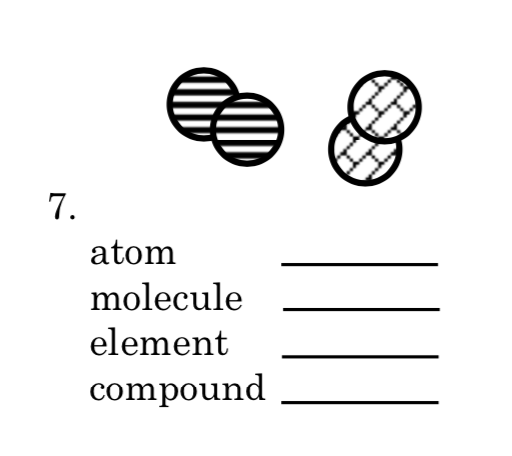
Atom=3
Molecule=1
Element=2
Compound=0
When heat is added to gas, the gas _________.
Expands
Draw a covalent bond of two hydrogen atoms using lewis dot structure
H-H
When water vapor gets cold, it turns to ________.
liquid
Orange Juice
liquid
Atom=9
Molecule=2
Element= 4 and 0
Compound= 3
Explain how a pressure rick cooker works
The boiling point of water increases so the pressure and temperature inside the cooker becomes very high.
Draw a covalent bond of sodium chloride atoms using lewis dot structure
Na+ Cl-
Which one has greater thermal energy? Why?
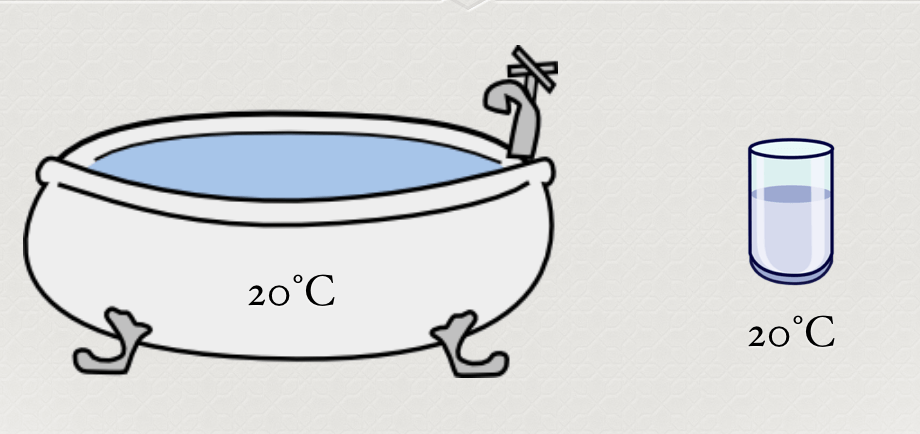
A cloud
solid, liquid and a gas
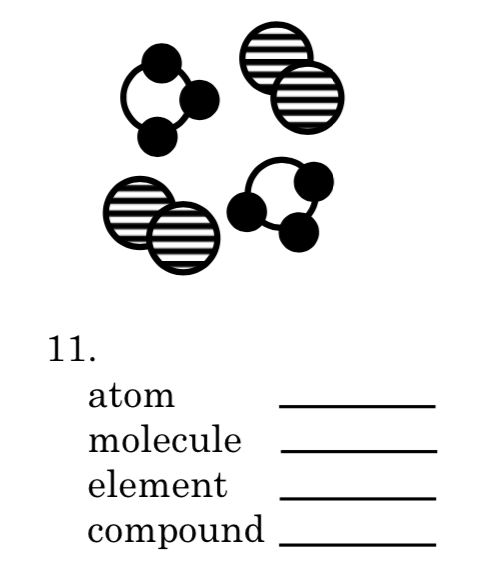
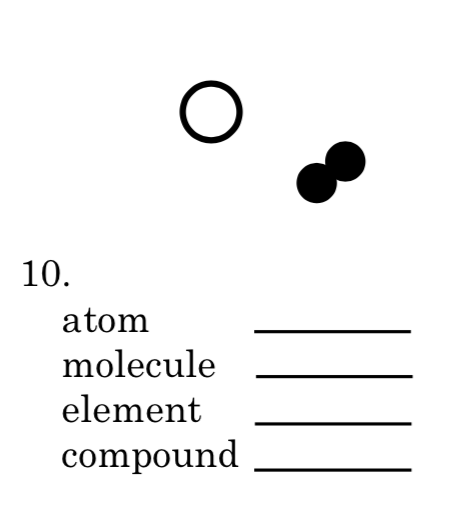
Atom=12
Molecule=4
Element=3 and 2
Compound=2
What is the order of electrons filling the shells? (5 shells)
2-8-8-18-32
What does the atomic mass represent in the periodic table?
Proton + Neutron
Which has more thermal energy? Pasta or water?
Water in pot = Higher thermal energy than pasta.
Because water is liquid and pasta is solid