Heat Transfer
anything that has mass and takes up space
What is matter?
This substance has a definite shape and definite volume.
What is a solid?
This results in a new substance and is permanently changed
What is a Chemical change?
K?
What is potassium?
2 or more atoms bonded together.
What is a molecule?
The amount of thermal energy depends on the amount of what?
What is matter?
the amount of matter something has
What is mass?
Heat transfer that does not need matter to transfer.
Radiation
A change in matter that doesn't create a new substance
What is Physical change?
This is the simplest form of matter that has a unique set of properties.
What is an element?
H20 (water) and CO2 (carbon dioxide) are examples of this as they are made up of different elements bonded together.
What is a compound?
This type of heat transfer only occurs in liquids and gases.
What is convection?
The amount of space an object takes up.
What is Volume?
DOUBLE JEOPARDY!
What is the particle motion in all 3 basic states of matter?
Magnetivity, Conductivity, and Boiling point
What is a physical property?
The first element on the periodic table is hydrogen. Name its atomic symbol.
What is H?
Describe H2O in terms of its components (parts)
What is 2 hydrogen atoms bonded with one oxygen atom?
Transfer of energy by direct touch
What is conduction?
A way of organizing all the elements so scientists can study matter.
What is the periodic table?
This substance takes the shape and volume of the container.
What is a gas?
The size of an object or the amount of space matter occupies?
What is volume?
How many neutrons does Sodium have?
What is 12?
Atoms packed closely together in this state and they vibrate in place
What is a solid?
Is this an example of a (Chemical or Physical) chang?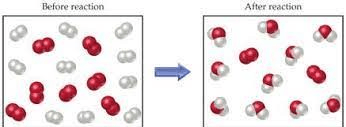
Chemical the particles are forming new materials (rearranging in different ratios).
this is the relationship between mass and volume
What is density?
Particles in this state of matter are loosely packed and slide past each other
What is a liquid?
Fills the space of the container
What is a gas?
How do you calculate the number of neutrons?
Atomic Mass - Atomic Number
How many atoms in one molecule of NaCl?
What is 2?
When thermal energy increases what happens to the particles?
What is speed up and expand?
All matter is made up of this.
What is atoms?
Give an example of convection
examples - ocean currents, water in a pot on the stove, air currents/wind
A measure of an objects mass divided by its volume? How much matter is in a given space?
What is density?
If Boron has 5 protons, how many electrons does it have?
Name the elements and identify the number of each element in CO2
1 carbon, 2 oxygen
What happens to the density of the cylinder if the piston moves from position A to B?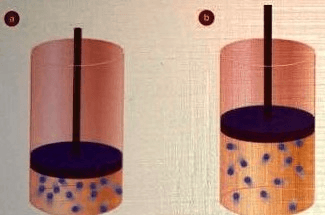
The gas would become less dense because there is more space to occupy. Less particles per unit of space.
Pure substances are ______ and compounds.
What are elements?
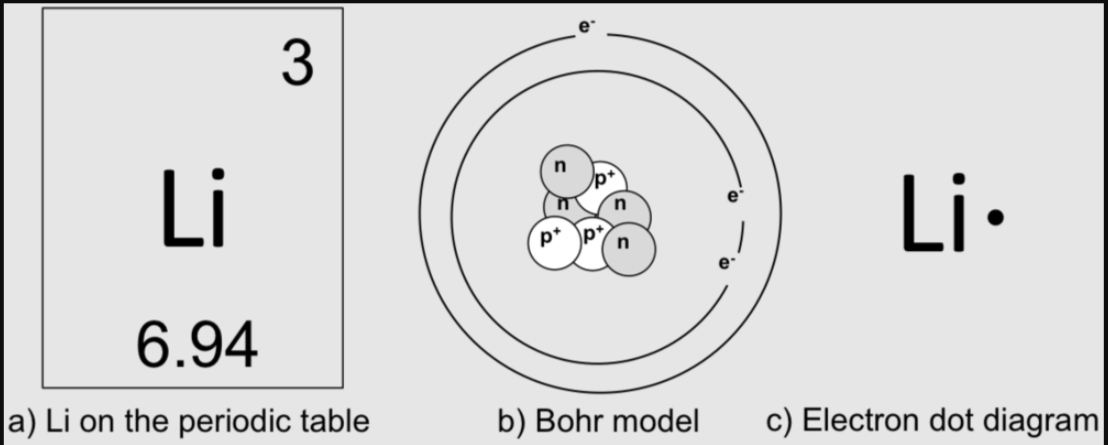
For letter C why is there only one dot?
Lewis dot shows only valence electrons.
There are two electrons in the first ring, lithium has 3 total. 1 remains and is the only valence electron.
Calculate the density of an object that has a mass of 25 g and a volume of 5 cubic cm.
What is 5 g/cm cubed?
How many electrons can fit in the first shell?
What is 2?
Same type of atoms bonded together form a(n)
What is element?
Hot air rises, cold air sinks - wind
What is convection?