Chromatography uses this property of a substance to separate mixtures.
What is solubility?
What is boiling point?
This results in a new substance and is permanently changed
What is a Chemical change?
The atomic number of Carbon is _______.
What is 6?
A substance containing 2 or more different atoms, bonded together is a
What is a compound?
Is Chromatography an example of a chemical change or physical change?
What is a physical change.
Is Distillation a Physical or Chemical Change?
What is Physical Change?
A change in matter that involves a change in a physical property but doesn't create a new substance
What is Physical change?
This particle defines an element.
What are protons?
Atoms of only one type is called ______________
an element
If you started with a black marker dot and as it tracked up the page into red and pink colors was this a pure substance?
What is no, its a mixture.
What solution did we distill?
What is a Sodium Chloride solution?
Which change occurs when you bake cookies?
What is Chemical change?
The number of protons and the number of _______ in an atom are equal.
What are electrons?
If a molecule has 1 Carbon to 4 Hydrogens how would you write it out?
What is CH4
If a marker dot didn't move up the paper, this tells you that the substance is:
What is not soluble in the liquid used.
What chemical did we use to detect if there were chloride ions in our finished product?
What is Silver Nitrate, AgNO3
Melting wax, ice, and metal are all what type of changes
What are physical changes?
The mass of an atom can be found by adding the number of protons and the number of _________.
What are neutrons?
How many Oxygen atoms are in 3 molecules of AgNO3
What is nine?
If a marker dot has stayed the same color as it tracks up the page then you know this to be a:
What is a pure substance.
Were there chloride ions detected in the sample that we did distill?
what is no.
When an egg is cracked and then cooked there is a physical change and a chemical change that occurs-name both.
What are Physical change-cracking the egg and chemical change-cooking?
How many neutrons are in an atom of iron (Fe) if the mass is 56?
What is 30?
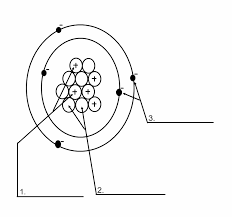
What are the parts of the atom and their charges?
What is 1. proton (positive), 2. neutron (neutral), 3. electron (negative)?