Everything that has mass and takes up space
What is matter?
What is homogenous?
Same Throughout
What are thr units of density?
g/ml
g/cm3
The type of change that occurs when a new substance is produced and cannot change back
What is a chemical change?
Crushing a can is an example of ______ change.

What is physical change?
Is a solution a mixture or a pure substance?
Mixture
The amount of matter in something
What is mass?
What is heterogenous?
See different parts.
Two liquids are poured into a cup, liquid A sinks and liquid B floats on liquid A, which is more dense?
liquid A
The type of change that happens but it's still the same substance
What is a physical change?
Burning wood in a campfire is _______ change.

What is a chemical change?
Saltwater is a mixture or a pure substance?
mixture
The amount of space something takes up
What is volume?
A salad is an example of which type of mixture?
Heterogenous
What is the formula for density? D=?
M/V
When a glass breaks, which change has occurred?

What is a physical change?
Fruit rotting is a ______ change
chemical
C6H12O6
mixture or pure substance?
If PS: element or compound?
If M: Homogeneous or heterogeneous?
pure stubstance
compound
Everything in the universe is matter and _______
What is energy?
What is space/vacuum?
A smoothie is an example of which type of mixture?
Homogenous
5 g of a substance has a density of 1.25 g/ml, what is the density of 100 g of the substance?
1.25 g/ml
Cake baking is an example of a ______ change.
What is chemical?
Oil floating on water is an example of different ____________.
What is densities?
Element or compound?
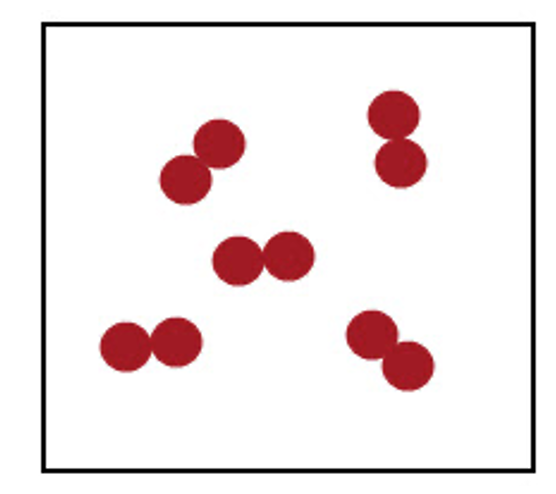
Element
Water displacement
What is the way to find the volume of an irregular solid?
A solution is ALWAYS what type of mixture?
Homogenous
Which is more dense, 1 gram of iron or 10 grams of styrofoam?
1 gram of iron
Name the 4 chemical changes.
Something: combusts, burns, digests, decomposes, reacts, cooks, bakes, etc.
Name a physical change of water
evaporate
boil
freeze
melt
condense
Mixture or Pure Substance of what?
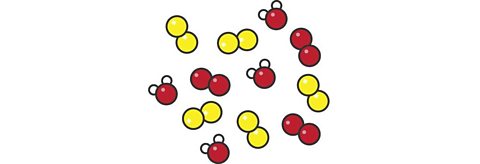
Mixture of elements and compounds