The smallest amount of a substance before it is no longer that substance.
Atom
What are the three parts of an atom?
protons. neutrons and electrons
What is the symbol for Hydrogen?
H
Why is air considered a mixture and not a compound?
A. It contains only one type of molecule
B. It cannot be separated
C. The gases are not chemically bonded
D. It reacts chemically to form oxygen
C. The gases are not chemically bonded
The number in front of a variable or element is called a_____?
Coefficient
How many elements are currently on the periodic table?
118
The physical form in which a substance exists at room temperature.
State of matter
Proton +, Neutron neutral, electron -
What is the symbol for gold?
Au
A substance that is dissolved in another substance, called the solvent, to form a solution
Solute
The number written slightly below to the right of a chemical symbol that shows how many atoms of an element are in a compound.
Subscript
What part of the periodic table is in blue?
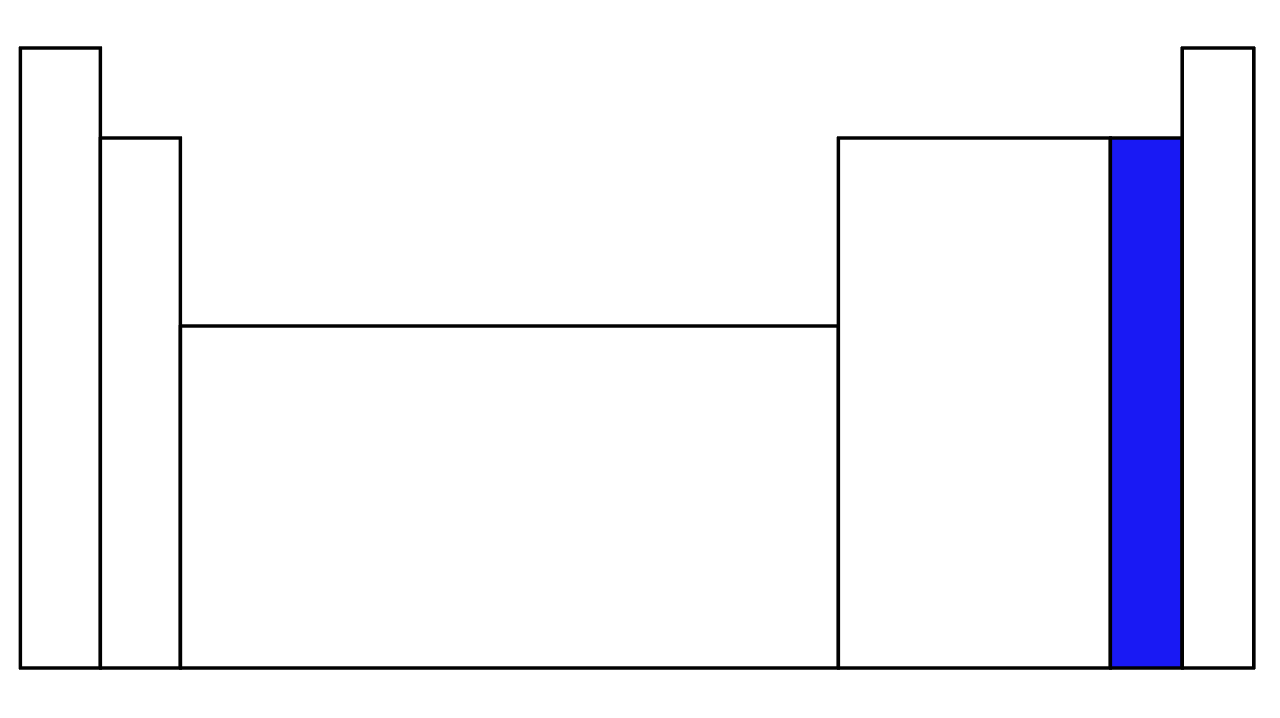
Halogens
Anything that has mass and takes up space.
Matter
What is the weight of a proton, neutron and electron?
Proton 1u
Neutron 1u
electron .0005u
What element does this sysmbol represent? K
Potassium
A mixture in which the different substances are not evenly distributed throughout is called a:
Heterogeneous mixture
How many atoms are on the reactant side of this chemical equation?
AgNO₃ + NaCl → AgCl + NaNO₃
7
Ag = 1
N = 1
O = 3
Na = 1
Cl = 1
What part of the periodic table is in blue?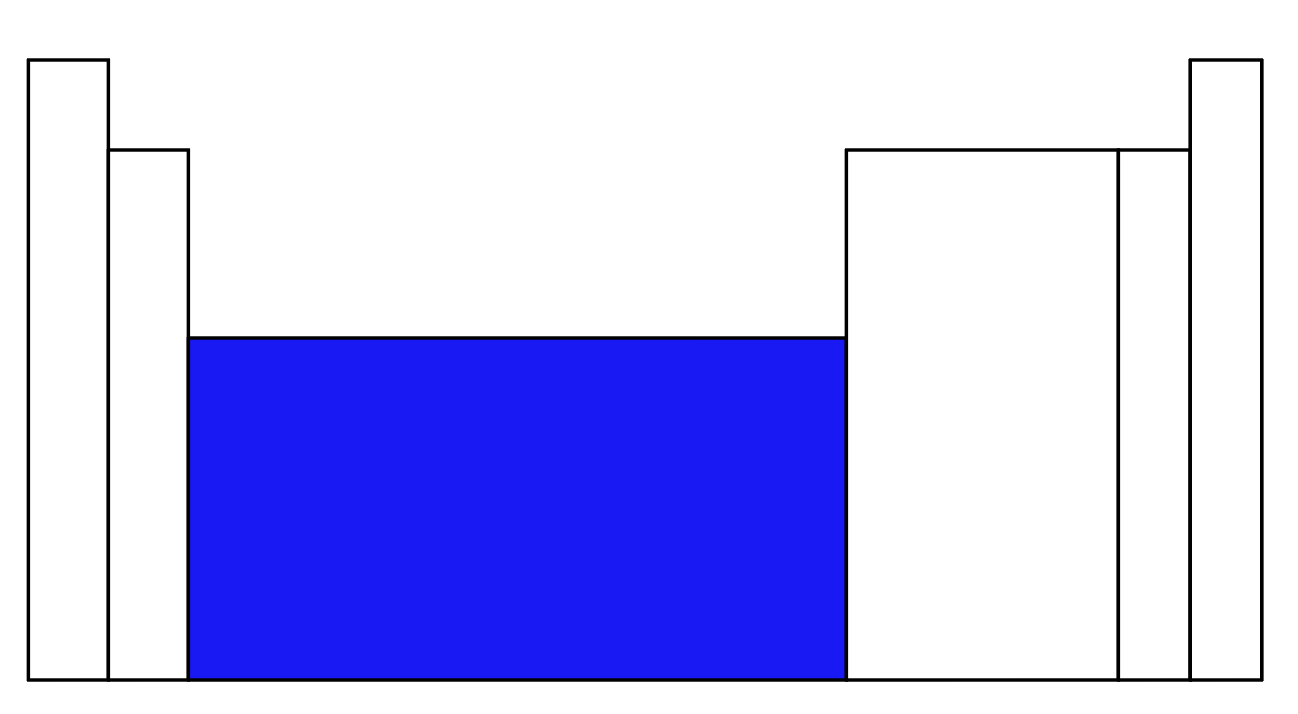
Transition Metals
The ability to be drawn or pulled into a wire.
Ductility
A group of atoms bonded together is called a____?
Molecule
What symbol does this symbol represent? Cl
Chlorine
A mixture in which the different substances are evenly distributed throughout is called a:
Homogeneous mixture
What is the right side of a chemical equation called?
Product
What is the blue part of the periodic table called?
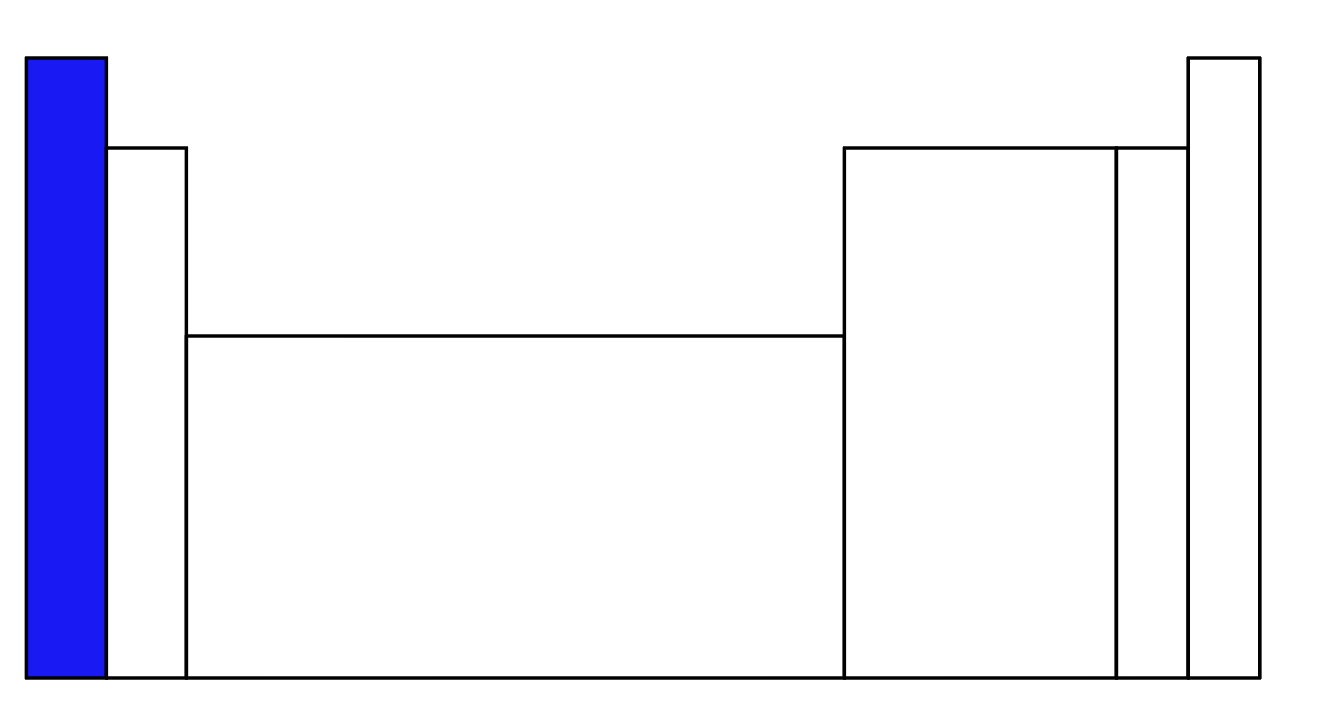
Alkali Metals
The electrons in the outermost shell of an atom.
Valence Electrons
An atom that has too many or too few electrons and does not have a neutral charge is called an ___?
Ion
What is the symbol for copper?
Cu
A substance in its purest form is called an____?
Element
For everyone!!
Bonus Question - Double Points:
Is this chemical equation balanced? If not, balance it
___Cs + ___N2 ----> _____Cs3N
No
6 Cs + ___N2 ----> 2 Cs3N
Who was the first person to develop/put the periodic table together?
Dimitri Mendeleev