Which subatomic particles are in the nucleus of the atom?
Protons and Neutrons
True or False: The Eiffel Tower has more volume in the summertime.
True: The Eiffel Tower takes up more space in the summer, because the particles in the metal spread out from the heat.
What are the states of matter?
Solid, Liquid, and Gas
What is the first step of the scientific method?
Make an observation
True or False: On the periodic table, periods are considered our columns.
FALSE: Periods are rows!!
What is thermal energy?
Thermal energy is heat. It causes our particles to move faster and spread out.
According to our matter definition, matter is anything that has _________ and takes _______
MASS and TAKES UP SPACE (VOLUME)
When atoms make bonds, what is being shared or given between the atoms?
Electrons!
How do we calculate atomic mass?
Protons + Neutrons
What is the 54 called? How many protons and electrons would we have?
54 is the atomic number!
Remember, the atomic number = protons
Protons=Electrons
54 Protons, 54 Electrons
You accidentally drop your ice cream cone on the ground. Why does it start to melt?
The ice cream is colder than the outside temperature. As the ice cream sits on the warmer ground, the heat from the sun and the ground speeds up the solid particles and makes them move faster causing the ice cream particles to spread out. This is why we would have a sticky ice cream puddle on the ground!
Draw out CO2 as a molecule. Is it a simple or extended structure?
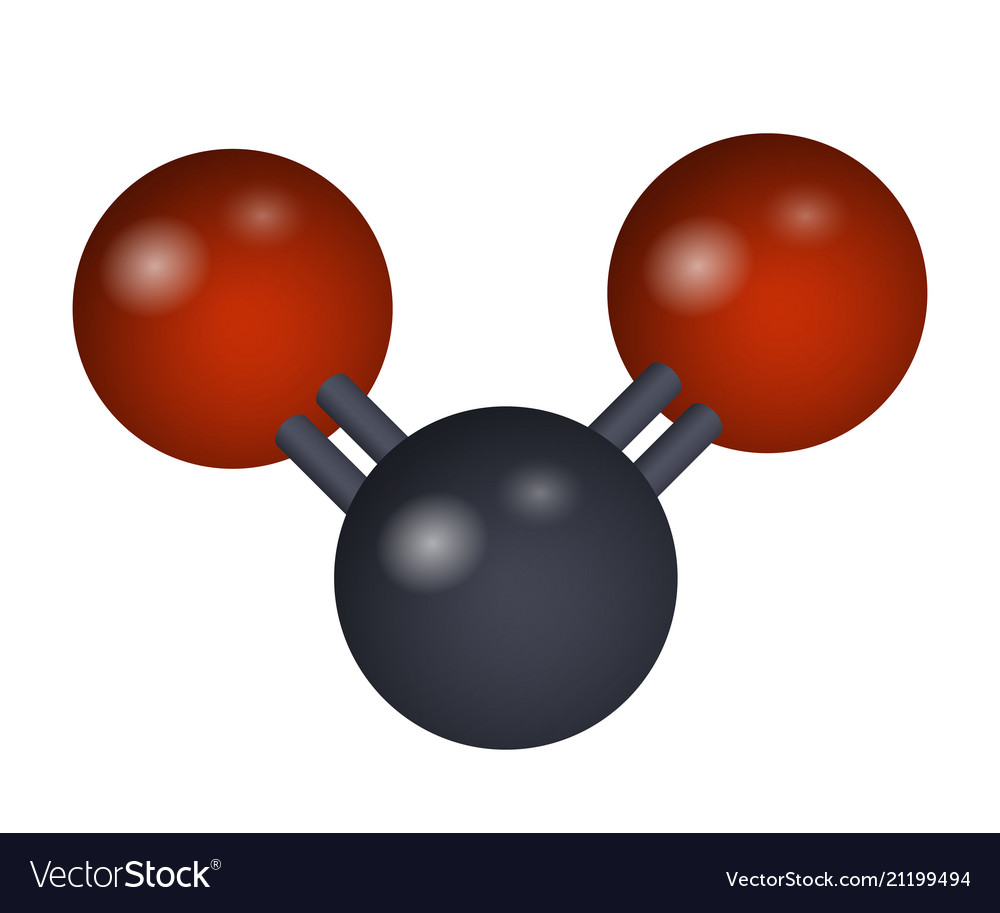 Extended-there is a double bond
Extended-there is a double bond
How do we find the amount of valence electrons for an element on the periodic table?
We need to count the group that the element is in. We skip over the transition metals so it can have between 1-8 valence electrons.
I decide that I need to make ice cubes for my water bottle so I put water in my freezer. Once it freezes, is the mass of the water going to increase, decrease, or stay the same?
According to the Law of Conservation, matter cannot be created or destroyed but only changes forms. In this example, the water (liquid) is being changed into ice (solid). The water amount stays the same.
What happens to the particles when a liquid turns into a solid?
-the particles move closer together, they get colder, they move slower and have less energy
What is the difference between SO3 and SO4?
Both of the compounds have 1 Sulfur
SO3 has 3 Oxygen atoms while SO4 has 4 Oxygen atoms
On a Bohr Model, how many maximum electrons can I have on my 1st energy shell/ring?
Maximum of 2
Why do people stress out about gaps in a bridge?
They do not understand that the gaps in the bridge cause the bridge to expand when it is warm in a given direction. Without the gaps, the bridge would expand in all directions and could break.
List the states of matter in terms of smallest energy to the most energy?
Solid (vibrate but not much movement)
Liquid (can flow past each other)
Gas (move very fast)
What is the difference between a Bohr Model and a Lewis structure?
Bohr models show us all of the electrons on energy shells/rings
Lewis structures show us only the valence electrons with the element´s symbol