What state of matter is this?
Liquid
What is reflection?
A reflection is created when light hits the surface of a solid or liquid and changes direction.
What is a mixture?
A mixture is a substance made up of two or more types of matter.
Decide if the following statement is true or false. If false, correct it.
Mixtures are chemical changes and can be separated.
False.
Mixtures are physical changes and can be separated.
Decide if the following statement is true or false. If false, correct it.
Chemical changes occur when one or more substances combine to form a new substance. These types of changes are reversible.
False.
Chemical changes occur when one or more substances combine to form a new substance. These types of changes are non-reversible.
List three examples of chemical reactions.
Three examples of chemical reactions are combustion, oxidation, and fermentation.
What state of matter is this?

Solid
What is diffusion?
When light hits a rough surface, the irregular reflection created is called diffusion.
Decide if the following statement is true or false. If false, correct it.
Mixtures are physical changes and can be separated into their original components.
True.
When do we use the process, or technique, of distillation?
A. Distillation is used to separate an insoluble solid from a mixture.
B. Distillation is used to separate a soluble solid from a solution.
C. Distillation is used to separate a liquid from a solution, or two liquids.
/chemistry-distillation-58aef7d15f9b58a3c92007fa.jpg)
C. Distillation is used to separate a liquid from a solution, or two liquids.
Which of these statements explains exothermic reactions?
A. Chemical reactions that produce, or create, thermal energy.
B. Chemical reactions that absorb, or take in, thermal energy.
A. Chemical reactions that produce, or create, thermal energy.
What are two negative effects of combustion?
Combustion contributes to global warming, pollution, and acid rain that can kill plants and ruin buildings.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.
Is this surface rough or smooth? Is this reflection or diffusion?

The surface of the lemons is rough, creating a diffusion of light, not a reflection.
Is this a mixture? If yes, what type of mixture is it?

What type of separation technique is shown below?
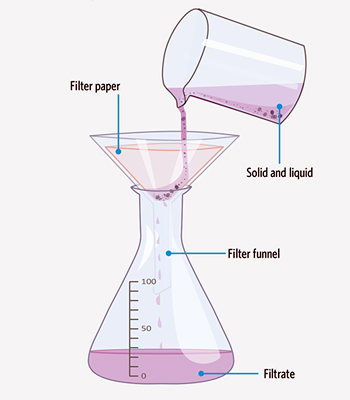
Filtration
This car is metal. It is experiencing oxidation and it is rusting. What are two effects of a metal rusting?

The metal loses its electrons, it changes color, and it becomes brittle, or weak.
Decide if the following statement is true or false. If false, correct it.
Combustion is an example of an endothermic reaction, because it produces thermal and light energy.
False.
Combustion is an example of an exothermic reaction, because it produces thermal and light energy.
What are the two ways matter can change?
Matter can change physically and chemically.
Decide if the following statement is true or false. If false, correct it.
Matter that is in a gaseous state has a smooth, reflective surface.
False.
Matter that is in a gaseous state is transparent and doesn't reflect light.
Matter that is in a liquid state has a smooth, reflective surface.
What is the difference between homogeneous and heterogeneous mixtures?
Homogeneous mixtures have a consistent color and texture with every part having the same properties.
Heterogeneous mixtures can have different colors and textures with every part maintaining its properties.
Dissolution can be used to separate a soluble solid (i.e., salt) from another insoluble solid (i.e., sand). What other separation techniques does dissolution use during the process?
A. Filtration and Evaporation
B. Distillation and Evaporation
C. Filtration and Distillation
A. Filtration and Evaporation
What happens to atoms in chemical reactions?
Atoms that make up the components are rearranged to create new atoms.
What type of chemical reaction is this? What is happening to the apple's electrons?
This is an example of oxidation. When the apple is exposed to the air, it loses its electrons.
Give an example of how human activity can chemically change matter.
HINT: Think of food.
Cooking is an example of how human activity can chemically change matter.
Digestion is an example of how human activity can chemically change matter.
Name the three parts that make up an atom and their charges (positive, negative, etc.).
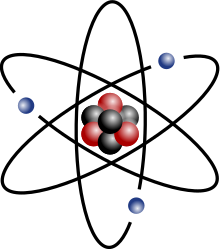
Protons - positively charged
Neutrons - no charge, neutral
Electrons - negatively charged
Solvent - the liquid component of a solution
Solute - the other components of a solution that dissolve into the solvent
Describe evaporation. When do we use it?
We use evaporation to separate a soluble solid (i.e., salt) from a solution (i.e., water). In order to separate the salt from the water, we must heat up the water until it turns into a gas, or evaporates. We are then left with the salt.
What type of chemical reaction is this? What type of gas is being produced by the yeast in the dough?
This is an example of fermentation. The yeast is producing carbon dioxide which causes the dough to rise.
What is needed for combustion to take place? What is produced?
We need fuel (wood, coal, or natural gas) and oxygen for combustion to take place. Combustion produces fire, smoke, and thermal energy.