In which of the following media - solids, liquids, gas, and vacuum - can sound waves be transmitted?
solids, liquids, and gas
Pink offspring are produced when a red bloom mates with a white flower. This is an illustration of:
A. Codominance
B. Heterodominance
C. Complete dominance
D. Incomplete dominance
D. Incomplete Dominance
What would the name of the parent root of the molecule below be if all prefixes were removed?
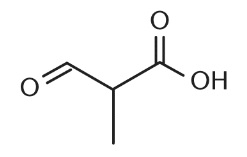
A. Propanol
B. Propanoate
C. Propanoic acid
D. Anhydride of propionate
C. Propanoic Acid
States that the number of moles of a gas present is proportional to its volume, assuming constant pressure and temperature.
Avogadro's Principle
Joy lives in a community where 40% of the residents are obese. Nearly half of the adults in her area are food stamp recipients and unemployed. Within ten blocks of Joy's home, there are no grocery stores but lots of fast-food restaurants. The community around Joy is in the following socioeconomic conditions:
I. a food desert
II. extreme poverty
III. relative poverty
A. I only
B. II only
C. I and II
D. I and III
D. I and III
A man moves 30 meters east, then 40 meters north. What is the distinction between his displacement and his trip distance?
20 meters
This states that all living things are composed of cells; cells are the basic functional unit of life; cells arise only from pre-existng cells; and cells carry their genetic information in the form of DNA.
Cell Theory
The carbon and nitrogen atoms in CN- have the following hybridizations:
A. sp3 and sp3, respectively
B. sp3 and sp, respectively
C. sp and sp3, respectively
D. sp and sp, respectively
D. sp and sp, respectively
Why do halogens and alkaline earth metals frequently form ionic bonds?
A. The halogens and alkaline earth metals in the same row have different atomic radii.
B. Compared to halogens, alkaline earth metals have substantially higher electron affinities.
C. Halogens and alkaline earth metals both create entire octets by sharing electrons equally.
D. Compared to alkaline earth metals, halogens have substantially higher electron affinities.
D. Compared to alkaline earth metals, halogens have substantially higher electron affinities.
A nation with a profit-driven, privately owned economy but state-funded public services like healthcare and education would be seen as:
A. socialist
B. capitalist
C. collectivist
D. welfare capitalist
D. welfare capitalist
A rock is dropped from a cliff that is 100 m above ground level. How long does it take the rock to reach the ground?
4.5 seconds
An element of secondary structure, marked by peptide chains lying alongside one another, forming rows or strands.
Beta-pleated sheet
Rank amine, carboxylic acid, aldehyde, and alkane in decreasing order of oxidation state.
A. Aldehyde, amine, alkane, carboxylic acid
B. Carboxylic acid, aldehyde, amine, alkane
C. Carboxylic acid, amine, aldehyde, alkane
D. Alkane, amine, aldehyde, carboxylic acid
B. Carboxylic Acid, Amine, Aldehyde, Alkane
Which of the following activities DOES NOT change a reaction's equilibrium state?
A. Increasing or decreasing heat.
B. Changing the amount of a catalyst.
C. Altering the volumes of the reactants.
D. Altering the concentrations of the reactants.
B. Changing the amount of a catalyst
All of the following socializing techniques MAY be used by adult prison systems to attempt to alter the conduct of inmates, EXCEPT:
A. resocialization
B. primary socialization
C. secondary socialization
D. anticipatory socialization
B. primary socialization
Law that states that the voltage drop across a resistor is proportional to the current flowing through it.
Ohms Law
Which of the following claims about nonpolar R groups in an aqueous solution is most likely accurate?
A. They hide inside proteins and are hydrophilic.
B. They are hidden within proteins and are hydrophobic.
C. They are present on protein surfaces and are hydrophilic.
D. They are present on protein surfaces and are hydrophobic.
B. They are hidden within proteins and are hydrophobic
Why does the equilibrium between enol and keto tautomers lean heavily toward enol?
I. The keto form has greater thermodynamic stability.
II. Enol is a lower energy form.
III. The enol form has a higher thermodynamic stability.
A. I only
B. III only
C. I and II only
D. II and III only
A. I only
Similar charges repel one another whereas opposite charges attract one another. How is it possible for positively charged protons to dwell in the nucleus?
A. Hadrons do not interact with one another through forces.
B. An opposite force is created by the electron cloud in the area.
C. The nuclear force is more powerful than the forces that repel protons.
D. The nucleus' neutrons keep the protons from interacting with one another.
C. The nuclear force is more powerful than the forces that repel protons.
What part of the middle ear produces vibrations that correspond to the sound waves hitting it?
A. Cochlea
B. Malleus
C. basilar membrane
D. tympanic membrane
D. tympanic membrane
How do the mass and proton numbers of a substance change when it undergoes alpha decay?
Mass number decreases by 4 and proton number decreases by 2.
The "triple helix" structure of collagen is made up of three carbon-based helices that are tightly coiled around one another. Which of these amino acids is most likely to be present in collagen in the largest amounts?
A. Proline
B. Glycine
C. Cysteine
D. Threonine
B. Glycine
In an IR spectrum, each of the following compounds will show absorption peaks EXCEPT:
A. O2
B. SO3
C. CO
D. HClO4
A. O2
The energy of a photon is determined by the researchers to be 3.3 x 10-12J during an experimental trial. What is the wave's frequency?
(Note: The value of Planck's constant is 6.6 x 10-34)
A. 2.0 x 1022
B. 1.5 x 1022
C. 1.0 x 1022
D. 0.5 x 1022
D. 0.5 x 1022
Which of the following statements about bipolar disorders is true?
I. If any, their genetic inheritance is minimal.
II. They are linked to higher serotonin levels in the brain.
III. A diagnosis of any of them requires at least one depressed episode.
A. I only
B. II only
C. I and III
D. II and III