The number 100100 has this many significant digits.
What is 4?
This term is used to describe when a measurement is very close to an expected standard quantity.
What is accuracy?
The name of this object.

What is a Graduated Cylinder?
The formula for density.
What is
D = M/V
The density of an object that has a mass of 23.45 g and a volume of 10.7 mL.
What is 2.19 g/mL?
The number of decimal places you can estimate when making a measurement.
What is 1?
The abbreviation for centimeters.
What is cm?
The number 0.00100 has this many significant digits.
What is 3?
You need this to determine if data is accurate?
What is a standard?
The name of this object.
What is an Erlenmeyer Flask?
How to find mass when given density and volume.
What is
The mass of an object that has a volume of 32.6 mL and a density of 1.39 g/mL.
What is 45.3 g?
The volume in this graduated cylinder.
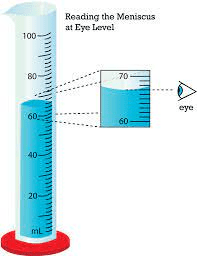
What is 67.0 mL?
The number of millimeters in a meter.
What is 1000?
The number 1001000.00 has this many significant digits.
What is 9?
This term could be used to describe a measured value with lots of significant figures.
What is precise?
You would you use this piece of lab equipment to get a precise measurement of the volume of a liquid.
What is a Graduated Cylinder?
The unit for density.
What is g/mL or g/cm3?
The density of a cube that measures 2.3 cm on each side and has a mass of 14.5 g.
What is 1.19 g/cm3?
The volume of the rock.
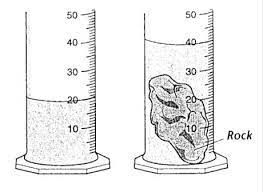
What is 20.0 mL?
The number of centimeters in 2.34 km.
What is 234,000 cm?
The answer to 10.00 x 1200 is reported with this many significant digits.
2
This term, or terms, could be used to describe this picture.
What is accuracy and precision?
You might use this piece of equipment to hold solids or liquids, mix substances, make rough measurements of volume, but would not be able to seal it with a rubber stopper.
What is a Beaker?
How to solve for volume when given density and mass.
What is
V = M/D
The density of an object that has a mass of 42.83 g and is placed into a graduated cylinder that originally had 25.0 mL of water in it. After you place the object into the cylinder, the water level rises to 42.1 mL.
What is 2.50 g/mL?
The part of the meniscus that is measured.
What is the bottom?
The larger of 23 cm or 0.023 m.
The answer to the following calculation is reported with this many significant digits.
(3.0 * 0.002) / 1000.
What is 1?
This dataset is the most precise of the group.
A: 12.45 g, 12.48 g, 12.39 g
B: 0.004 L, 0.009 L, 0.001 L
C: 27.001 m, 27.004 m, 27.002 m
What is dataset C?
You would use this piece of equipment to mix solutions with a specific concentration.

What is a Volumetric Flask?
The unit of the answer when you divide mass by density.
What is mL or cm3?
The density of 45.3 mL of solution that has a mass of 108.45 g when poured into a graduated cylinder that has a mass of 65.42 g empty.
What is 0.950 g/mL?
The number of decimal places reported when measuring centimeters with this instrument.
The number of kilometers in a meter.
What is 0.001?