What are alloys?
A mixture of metals OR a mixture of metals and nonmetals.
A BOND that is formed between only metals, specifically one type of metal.
Metallic Bond
REVIEW: Atoms get LARGER as we go in which two directions on the periodic table?
To the LEFT and DOWN.
A Nickel (worth 5 cents) is a mixture of 25% Nickel (Ni) and 75% Copper (Cu). Draw a diagram to represent this alloy.

Blue = Nickel (Ni)
Red= Copper (Cu)
***Since this mixture is made up of more Copper (Cu) than Nickel (Ni) you want more Copper (Cu) ions showing than you do Nickel (Ni) ions. The exact amount doesn't matter, as long as you have more Copper (Cu) ions than Nickel (Ni) ions. These ions should be the same size also! Since this is a substitutional alloy.

Aluminum (Al) foil is a bond between many Aluminum (Al) ions. This would be considered a what?

A Metallic Bond
True or False: A mixture between a metal and a nonmetal is an ionic bond. EXPLAIN!
FALSE! A BOND between a metal and a nonmetal is an ionic bond. However, a mixture between a metal and a nonmetal is called an alloy.
True or False: Metallic Bonds are a mixture between metals. EXPLAIN!
FALSE! Metallic Bonds are a bond between metals NOT a mixture, that is an alloy.
A cast iron skillet is a mixture of Iron (Fe), Carbon (C), and some other elements. This would be considered a what? EXPLAIN!

An Interstitial Alloy, because it is a mixture and NOT a bond, making it an alloy and NOT a Metallic Bond. It is an Interstitial Alloy because Iron (Fe) and Carbon (C) are different sizes.
Draw a diagram to represent the alloy of solder, which is a mixture of Tin (Sn) and Lead (Pb).

Blue = Tin (Sn)
Red= Lead (Pb)
***It doesn't matter which color you choose to represent which element NOR does the amount of each matter. As long as you are able to realize that these two elements are the same size (substitutional alloy) then you are good!

Remember when we saw the demo of PURE Potassium (K) reacting in water to create a mini explosion? What type of bond is PURE Potassium (K)? EXPLAIN!

Pure Potassium (K) is a Metallic Bond because it is a bond between only one type of metal and that metal in this case is Potassium (K).
What type of allow contains elements that are similar (same) in size?
Substitutional Alloys
The gif below is describing what about Metallic Bonds?

Sea of Electrons
A bond between 1 million Zinc (Zn) ions is a what? EXPLAIN!
A Metallic Bond, because there are multiple metals (specifically one type of metal) bonding together. Since it is NOT a mixture of metals it is NOT an Alloy.
An alloy made up of Zinc (Zn) and Carbon (C) would be considered as which type of alloy? EXPLAIN!
An Interstitial alloy, because Zinc (Zn) and Carbon (C) are different sizes. Zinc (Zn) is larger than Carbon (C).
Draw a diagram of the Metallic Bond of Copper (Cu).
Yellow = Copper (Cu) ions
Green = Free-Flowing (Delocalized) electrons.

What type of alloy contains elements that are different in size?
Interstitial Alloy
True or False: Metallic Bonds just like Ionic Bonds have electrons that are stationary and do not move. EXPLAIN!
FALSE! Unlike Ionic Bonds, Metallic Bonds have DELOCALIZED electrons, which makes them free-flowing.
A bond between 200 Copper (Cu) ions is a what? EXPLAIN!

A Metallic Bond, because there are multiple metals (specifically one type of metal) bonding together. Since it is NOT a mixture of metals it is NOT an Alloy.
This diagram represents what type of alloy?

An Interstitial Alloy
Sodium (Na) is a(n) _________________ bond when it is by itself. However, when it bonds with Chlorine (Cl) it becomes a(n) ________________ bond. Why is this?
-Metallic
-Ionic
When Sodium (Na) is by itself, it bonds with other Sodium (Na) ions. This is a bond between metals making this a Metallic Bond.
When Sodium (Na) bonds with Chlorine (Cl), it is a bond between a metal (Na) and a nonmetal (Cl) making this an ionic bond.
Identify each diagram.
Diagram 1:

Diagram 2:

Diagram 1 is a substitutional alloy
Diagram 2 is an interstitial alloy.
What makes Metallic Bonds so good at being conductive?
Their free-flowing electrons.
A penny is a mixture of Copper (Cu) and Zinc (Zn). This would be considered a what? EXPLAIN!
![]()
A Substitutional Alloy, because since it is a mixture and NOT a bond it is an Alloy and NOT a Metallic Bond. It is a Substitutional Alloy because Copper (Cu) and Zinc (Zn) are the same sizes.
Which diagram would best represent a mixture of Silver (Ag) and Silicon (Si)? EXPLAIN!
Diagram 1:

Diagram 2:
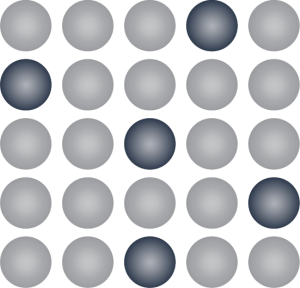
Diagram 1, because Silver (Ag) is much larger than Silicon (Si) making them different sizes. Therefore, this is an Interstitial Alloy.
True or False: Electrons in Metallic Bonds are attracted to the metals in the bond. EXPLAIN!
FALSE! Electrons in Metallic Bonds are free-flowing (delocalized a.k.a. sea of electrons) and therefore, are NOT attracted to any atom in the bond.
