Name two metals which are found in nature in free
state.
Gold and Silver
Most malleable and most ductile metal.
Gold or Silver (Any one)
Which of the following statements is true for an amphoteric oxide?
(a) It reacts only with acid and does not form water.
(b) It reacts with acid as well as base to form salt and hydrogen gas.
(c) It reacts with both acid as well as base to form salt and water.
(d) It reacts only with base and does not form water.
c
The electronic configurations of three elements X, Y and Z are X — 2, 8; Y —2, 8, 7 and Z — 2, 8, 2. Which of the following is correct?
A. X is a metal
B. Y is a metal
C. Z is a non-metal
D. Y is a non-metal and Z is a metal
Y is a non-metal and Z is a metal
Name a metal which is so soft, that it can be cut with a
knife and a non-metal which is the hardest known
substance.
Sodium or Potassium
Diamond (Carbon)
Only Lustrous Non Metals
IODINE
(Not Diamond)
Which one of the following statements is true about the position of metals
in the activity series of metals?
(a) Copper is below hydrogen but above lead.
(b) Iron is below lead and zinc.
(c) Zinc is below magnesium but above aluminium.
(d) Magnesium is below calcium but above aluminium.
d
Wat is the products formed when Iron reacts with water?
Sry Iron doesn't react with water, it reacts only with steam
Tell any 3 classification of metals
Alkali metals
Alkaline earth metals
Transition metals
Why?
(a) gold and silver are used for making jewellery.
(b) a few metals are used for making cooking utensils.
a) Lustrous
b) Good conductor
d
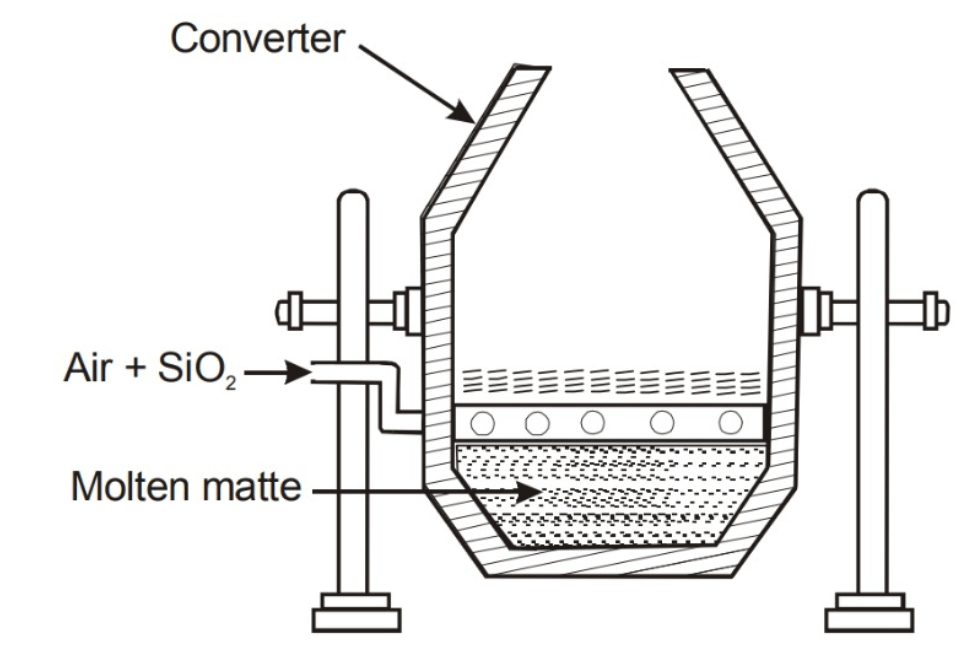
What does this image represent
Just Kidding Enjoiiii Free Points 600!!!!!
Why does Magnesium float on the surface of hot water?
A. It reacts with hot water to form magnesium hydroxide and hydrogen.
B. It reacts with hot water to form magnesium hydroxide and oxygen.
C. It reacts with hot water to form magnesium oxide and hydrogen.
D. It reacts with hot water to form magnesium oxide and oxygen.
A. It reacts with hot water to form magnesium hydroxide and hydrogen.
Which metal is poor conductor of heat
Lead
The pair(s) which will show displacement reaction is/ are
(i) NaCl solution and copper metal
(ii) AgNO3 solution and copper metal
(iii) Al2(SO4)3 solution and magnesium metal
(iv) ZnSO4 solution and iron metal.
(a) (ii) only
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (ii)
b
An element ‘A’ form two oxides AO and AO2. The
oxide AO is neutral whereas the oxide AO2 is acidic
in nature. Would you call element ‘A’ a metal or a
non-metal?
The element is carbon which is a non- metal. CO is
neutral and CO2 is acidic in nature.
How many naturally available elements are liquid at RT and name them
Mercury
Bromine
Which property of Al makes it useful in the field of packing materials.
Malleability
A student, took four metals P, Q, R and S and carried out different experiments to study the properties of metals. Some of the observations were:
- All metals could not be cut with knife except metal R.
- Metal P combined with oxygen to form an oxide
- M2O3 which reacted with both acids and bases.
- Reaction with water:
P- Did not react either with cold or hot water but reacted with steam.
Q-Reacted with hot water and the metal started floating
R- Reacted violently with cold water.
S - Did not react with water at all
1. Out of the given metals, the one which needs to be stored in kerosene is
(a) P (b) R (c) S (d) Q
2. Metal which forms amphoteric oxides is
(a) P (b) Q (c) R (d) S.
3. The increasing order of the reactivity of the four metals is
(a) P<Q<R <S (b) S<R<Q<P (c) S<P<Q<R (d) P<R<Q<S.
1. b
2. a
3. c
Aluminium is highly reactive metal but still used for making cooking utensils. Aren't humans afraid that Al might react with food and cause probs......... So why?
It forms an oxide layer on its surface which makes it passive.