What is the isotopic notation for an atom that has 4 protons, 3 neutrons, & 4 electrons.
74 Be
What are the columns & rows called on the periodic table?
Groups & Periods
What are the rules for naming the first and second element in a univalent binary ionic compound?
First Element Keeps it's name
Second Element keeps root, drops ending adds -ide.
Covalent compound consist between what type of elements?
The electrons will be what?
To obtain what rule?
Nonmetals
Shared
Octet
Which ion has the largest charge based on the graph provided?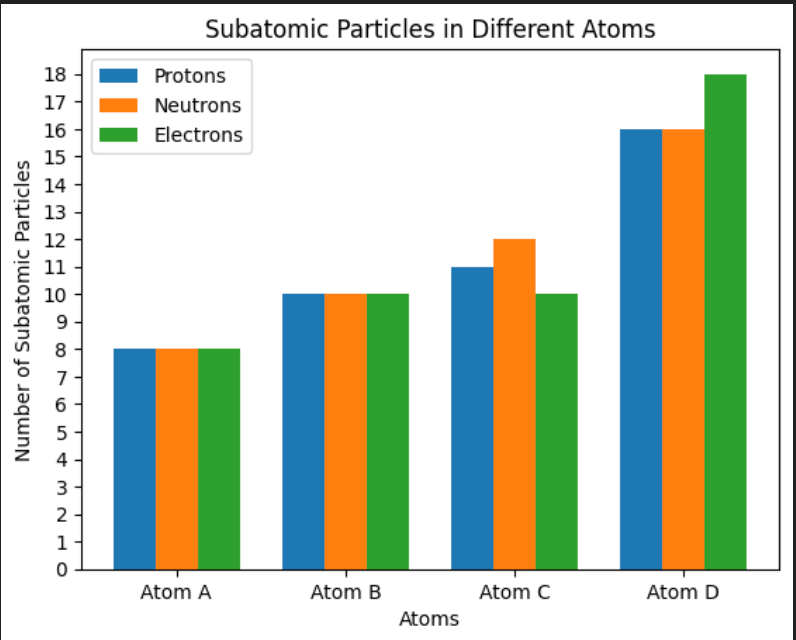
Ion (Atom) D
What are some of Dmitri Mendeleev's contributions to the development of the periodic table?
(Needs 2 Answers)
Predicted Properties of Elements
Organized the periodic table by mass
TRUE OR FALSE
Polyatomic ions are always written second.
False
NH4+
Diiodine Monoselenide
What is the noble gas configuration of an atom of phosphorus?
Which elements from the halogen family are diatomics, and form ions with a minus 1 charge?
Fluorine, Chlorine, Bromine, Iodine
What are the 4 main properties of an ionic compound?
Melting Point/Solubility/Conductivity in Water/Structure
High Melting Point
Soluble
Bright Bulb (Good Conductor)
Flakes/Crystals
Which substances are nonpolar?
CO2
BCl3
SCl2
O2
HCN
CO2
BCl3
O2
What is the order of the atomic models and the scientist who created them?
Dalton (Solid Sphere), Thomson (Plum Pudding), Rutherford (Nuclear Model), Bohr (Planetary), Heisenberg & Schrödinger (Quantum Mechanical)
What periodic trends cause reactivity to increase?
1. Increase atomic radius
2. Decrease in ionization energy & electronegativity
3. Increased electron Shielding
Name the following ionic compound
PbS2
Lead (IV) Sulfide
Identify the molecular shape with the descriptions below:
Three electron groups
Two groups are made of shared pairs
One group is made of a lone pair
Hint* There are three types
Alpha
Beta
Gamma
What two factors contribute to the trend in atomic radius across a period on the periodic table?
Increase number of protons & effective nuclear charge.
Draw the lewis structure for the ion Magnesium Bromide
I will draw the right answer
Draw the lewis structure for the molecule: CO2
What is the molecular geometry? Electron?
Is the molecule polar or nonpolar?
I will draw the structure
Linear
Nonpolar