What does PPE stand for?
Personal Protective Equipment
DOUBLE POINTS!!!
(1) What is Matter?
(2) What is it made up of?
(3) How many states are there?
(1) Anything that has mass and occupies space.
(2) Matter is made up of tiny particles called atoms.
(3) 3 States: Solid, liquid, and gas.
Name 3 Physical Characteristics
Phase of matter, Color, Hardness, Luster, Malleable, Ductile
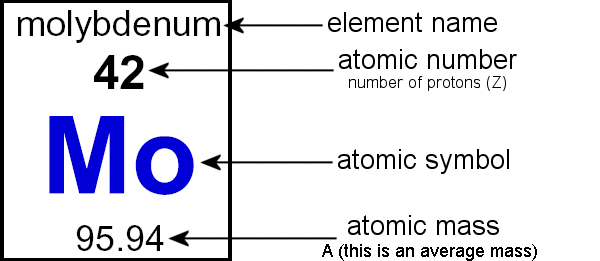
What is Atomic Mass and where can you find it on the Periodic Table?
Sum of protons and neutrons of a single atom.
Describe water displacement.
When an object is submerged underneath water in a container, the level of the water level rises.
If there was ever a fire what are 3 different safety equipments that could be used in the classroom?
2. Fire Blanket
3. Pull Down Shower
THE AMOUNT OF SUBSTANCE THAT MAKES UP AN OBJECT.
TOOL: TRIPLE BEAM BALANCE
UNIT: GRAMS
Name 2 Physical Changes
Freezing/ melting
Boiling/ Condensing
Evaporation
Cleavage/ fracture
What does the Atomic Number tell you?
Number of protons
Chemical Formula:
The first letter of an element’s symbol is always ___________.
If there is a second letter it is ___________.
(1) capitalized
(2) lower case
If you get something in your eyes where do you go?
Eye Wash Station
What is the Density Formula?
D= M/V
Name 3 Chemical Characteristics
Reactivity, Flammable, Toxicity, Radioactivity, Acidity/ Basicity
DOUBLE POINTS!!!
What is a chemical symbol?
What is the difference between and Element and a Compound?
A chemical symbol is a one- or two-letter designation of an element. Some examples of chemical symbols are O for oxygen, Zn for zinc.
An element is a pure substance made up of only one type of atom, a compound is a substance made up of two or more elements chemically combined in a fixed ratio.
DOUBLE POINTS!!!
(1) Who created the framework for the Periodic Table?
(2) How many elements are currently on the periodic table present day?
(1) Dmitri Mendeleev
(2) 118
True or False:
Should any accident occur, notify your teacher(s) first?
True
Define Volume, a tool used to measure it and its unit.
THE AMOUNT OF SPACE AN OBJECT OCCUPIES.
TOOL: RULER
UNIT: ML
DOUBLE POINTS!!!
What is the Law of Conservation?
Mass cannot be created or destroyed during a chemical reaction, but is always conserved.
What is the difference between a homogenous mixture and a heterogenous mixture?
Homogeneous mixtures are uniform in structure or composition. Homogeneous mixtures are typically those whose component parts cannot be easily separated. (Ex: salt water mixture)
Heterogeneous mixtures consist of distinct substances and don’t have a uniform composition. (Ex: salad is a heterogeneous mixture: you can see that a salad obviously consists of different, separate ingredients, such as lettuce, tomatoes, and carrots)
12
True or False:
After conducting a lab and mixing chemicals, when you are down you should dispose of the chemicals by pouring them down the drain.
False: Lab waste must be disposed of properly.
DOUBLE POINTS!!!
What is the Mnemonic Device used for prefixes of the Metric System?
Kids Have Dirty Mouths Lips Gums Drinking Chocolate Milk
Name 2 Chemical Reaction Indicators
Formation of gas
Formation of precipitate
Formation of odor
Emission of light
Changes in temperature
Change in color
Dalton's Model
J.J. Thompson Model (Plum Pudding)
Rutherford's Model
Bohr's Model
Dalton’s model: Dalton believed that atoms of an element have the same mass and that each different element had its own type of atom with different masses.
J.J. Thompson: discovered the first of the sub-atomic particles: the electron. Thompson thought that an atom looked like a ball of positive charge with electrons dotted through it, like plums in a pudding.
Rutherford: shows that an atom is mostly empty space, with electrons orbiting a fixed, positively charged nucleus in set, predictable paths.
Bohr: electrons orbit the nucleus in orbits that have a set size and energy. The energy of the orbit is related to its size. The lowest energy is found in the smallest orbit.
DOUBLE POINTS!!!
How is the Periodic Table of the Elements arranged?
Alkali metals: Group 1, the table's first column. Shiny and soft enough to cut with a knife, these metals start with lithium (Li) and end with francium (Fr). They are also extremely reactive and will burst into flame or even explode on contact with water.
Alkaline-earth metals: The alkaline-earth metals make up Group 2 of the periodic table, from beryllium (Be) through radium (Ra). Each of these elements has two electrons in its outermost energy level, which makes the alkaline earths reactive enough that they're rarely found alone in nature.
Lanthanides: This is the lanthanides, elements 57 through 71 — lanthanum (La) to lutetium (Lu). The elements in this group have a silvery white color and tarnish on contact with air.
Actinides: The actinides line the bottom row of the island and comprise elements 89, actinium (Ac), through 103, lawrencium (Lr). Of these elements, only thorium (Th) and uranium (U) occur naturally on Earth in substantial amounts. All are radioactive. The actinides and the lanthanides together form a group called the inner transition metals.
Transition metals: Groups 3 through 12 represent the rest of the transition metals. Hard but malleable, shiny, and possessing good conductivity, these elements are what you typically think of when you hear the word metal. Many of the greatest hits of the metal world — including gold, silver, iron and platinum — live here.
Post-transition metals: The post-transition metals are aluminum (Al), gallium (Ga), indium (In), thallium (Tl), tin(Sn), lead (Pb) and bismuth (Bi), and they span Group 13 to Group 17. These elements have some of the classic characteristics of the transition metals, but they tend to be softer and conduct more poorly than other transition metals. Many periodic tables will feature a bolded "staircase" line below the diagonal connecting boron with astatine.
Metalloids: The metalloids are boron (B), silicon (Si), germanium (Ge), arsenic(As), antimony (Sb), tellurium (Te) and polonium (Po). They form the staircase that represents the gradual transition from metals to nonmetals. These elements sometimes behave as semiconductors (B, Si, Ge) rather than as conductors. Metalloids are also called "semimetals" or "poor metals."
Nonmetals: Everything else to the upper right of the staircase — plus hydrogen(H), stranded way back in Group 1 — is a nonmetal. These include carbon(C), nitrogen (N), phosphorus (P), oxygen (O), sulfur (S) and selenium (Se).
Halogens: The top four elements of Group 17, from fluorine (F) through astatine(At), represent one of two subsets of the nonmetals. The halogens are quite chemically reactive and tend to pair up with alkali metals to produce various types of salt.
Noble gases: Colorless, odorless and almost completely nonreactive, the inert, or noble gases round out the table in Group 18.