Element - Sodium
Symbol - ________________
Atomic # - 11
Atomic Mass - 23
Protons - ________________
Neutrons - _______________
Electrons - ______________
Element - Sodium
Symbol - Na
Atomic # - 11
Atomic Mass - 23
Protons - 11
Neutrons - 12
Electrons - 11
metal or nonmetal?
Negative ions are usually __________________.
nonmetals
What is the chemical name of the compound, PbO2?
Lead (IV) Oxide
What element is in group IVA, period 5?
(Sn) Tin
What type of compounds uses the Greek prefixes?
covalent compounds
Element - _______________
Symbol - Y
Atomic # - _______________
Atomic Mass - 89
Protons - ______________
Neutrons - ______________
Electrons - ______________
Charge - -3
Element - Yttrium
Symbol - Y
Atomic # - 39
Atomic Mass - 89
Protons - 39
Neutrons - 50
Electrons - 42
Charge - -3
These are involve in chemical bonding?
Valence electrons
What is the formula of aluminum chloride?
AlCl3
Which element with the highest ionization energy:
Al Si P S
(S) Sulfur
What is the strongest intermolecular force?
Hydrogen Bond
Element - Lithium
Symbol - Li-10
Atomic # - _______________
Atomic Mass - ____________
Protons - 3
Neutrons - ______________
Electrons - ______________
Element - Lithium
Symbol - Li-10
Atomic # - 3
Atomic Mass - 10
Protons - 3
Neutrons - 7
Electrons - 3
Which type of bond shares electrons?
Covalent bond
What is the name of the compound, octaphosphorus heptoxide
P8O7
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d6
Ruthenium (Ru)
How can atoms become a cation?
lose electron
Element - Copper
Symbol - ________________
Atomic # - _______________
Atomic Mass - ____________
Protons - 29
Neutrons - 41
Electrons - ______________
Element - Copper
Symbol - Cu - 70
Atomic # - 29
Atomic Mass - 70
Protons - 29
Neutrons - 41
Electrons - 29
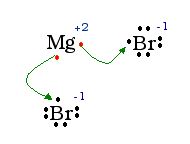
Show the Lewis Dot structure of MgBr2.
Give the formula of Iron (III) bromide.
FeBr3
Which correctly lists four atoms from smallest to largest atomic radii?
a. Cs, K, Rb, Na
b. K, Ca, Ge, As
c. Fr, Ba, Ga, Si
d. F, S, As, Sn
d. F, S, As, Sn
What is ionization energy?
the energy required to remove an electron from an atom
Element - Nitrogen
Symbol - N3+
Atomic # - _______________
Atomic Mass - 14
Protons - ________________
Neutrons - _______________
Electrons - ______________
Element - Nitrogen
Symbol - N3+
Atomic # - 7
Atomic Mass - 14
Protons - 7
Neutrons - 7
Electrons - 4
Show the Lewis Dot structure of CCl4.

What is the chemical formula of strontium phosphate?
Sr3(PO4)2
Write the electron configuration of Cesium (Cs).
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1
What law or principle states that each electron follows a sequence of atomic orbitals from lowest energy to highest energy
Aufbau Principle