This state of matter has particles vibrating in fixed positions.
What is a solid?
This is the energy stored in chemical bonds
What is potential energy?
A gas behaves most ideally under these conditions.
What are high temperature and low pressure?
This subatomic particle determines the identity of an element.
What is the proton?
Elements are arranged on the periodic table by this value.
What is atomic number?
Electrons are transferred in this type of bond?
What is Ionic?
The ability of zinc to react with hydrochloric acid is an example of this type of property.
What is a chemical property?
If energy appears on the reactant side of an equation, the reaction is described as this.
What is endothermic?
The boiling point of ethanoic acid at 60 kPa
What is 102-104oC ?
Has the same number of protons but a different number of neutrons.
What are isotopes?
The name of the group that contains elements with 2 valence electrons.
What are the Alkaline Earth Metals?
This bond is is the most polar:
A) C–H
B) O–H
C) N–N
D) Cl–Cl
What is B, O-H?
A liquid mixture of ethanol and water could be separated by this method which involves heating and condensing vapors based on different boiling points.
What is distillation?
Calculate the heat required to melt 25.0 g of ice at 0°C using the heat of fusion.
What is 8,350 J?
Convert the number of moles in 11.2 L of nitrogen gas at STP
What is 0.5 mol
Chlorine-37 has this many neutrons.
What is 20?
This group of elements are chemically inert.
What are the noble gases?
The reason water is a polar molecule.
What is water is asymmetrical?
Draw a particle diagram representing a mixture of an element and a compound in the gas phase, showing at least 6 particles of each substance.
[Teacher Check]
This is the heat absorbed when 75 g of water warms from 20°C to 40°C.
What is 6,270 joules?
Draw the relationship between pressure and volume.
[Graph shows inverse relationship]
It's how atomic spectra are produced
Electrons in the excited state fall back to the grounds state and release energy in the form of light.
It's why ionization energy increases across a period.
What is increased nuclear charge?
Draw a Lewis electron-dot diagram for MgCl₂
[Teacher Check]
This substance cannot be broken down by a chemical change:
A) H₂O
B) CO₂
C) NaCl
D) Ne
What is D, neon?
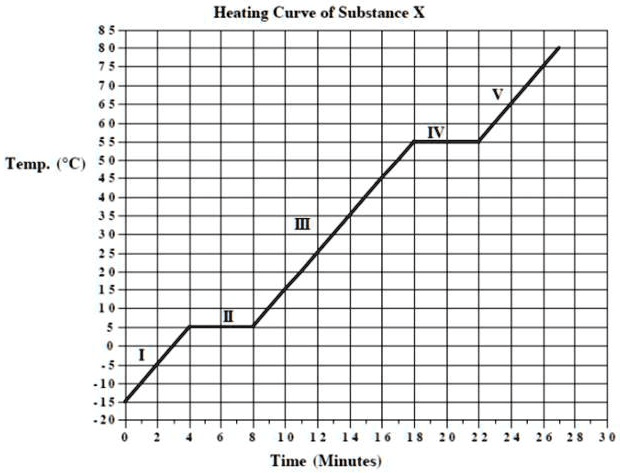
A heating curve shows a plateau from 4–8 minutes at 5°C. Identify the phase change AND explain what happens to kinetic and potential energy during this time.
What is melting?
Kinetic energy is constant
Potential energy increases
5.00 L gas is at 27oC and 75.0 kPa calculate the new volume at STP.
What is 3.37 or 3.4 L?
Calculate the weighted atomic mass.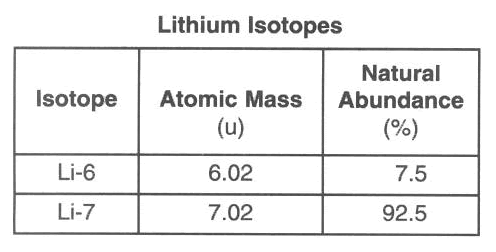
What is 6.945 u?
Explain why a Cl- ion has a larger radius than a Cl atom?
A Cl- ion has more electrons than a Cl atom.
*Cl- ions DO NOT gain electrons, atoms do
Identify the intermolecular force between NH₃ molecules
What are hydrogen bonds?