Ag2O
Silver (I) Oxide
SO
Sulfur Monoxide
NaCl
Sodium Chloride
Calcium Hydroxide
Ca(OH)2
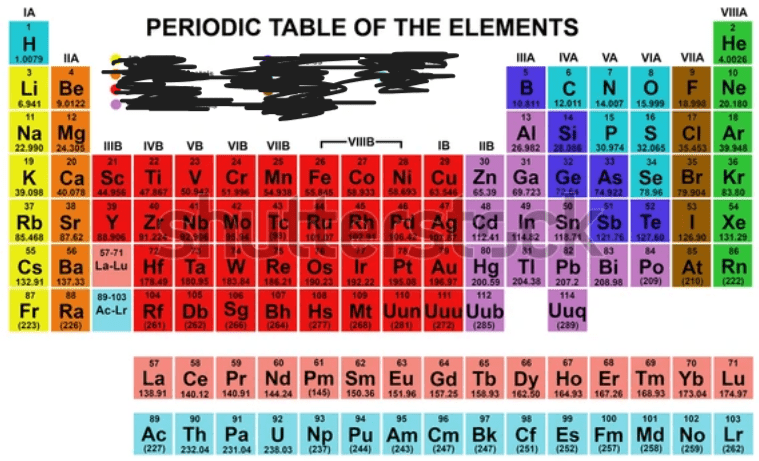
The Halogens are shaded in what color?
Brown
CrO
Chromium (II) Oxide
diphosphorus pentoxide
P2O5
Barium Fluoride
BaF2
Ba(ClO3)2
Barium Chlorate
Place the following elements in order of increasing ionization energy
Br, F, I
I, Br, F
Copper (I) Selenide
Cu2Se
C4S8
Tetracarbon Octasulfide
AlI3
Aluminum Iodide
Cu(C2H3O2)2
Copper (II) Acetate

The metalloids are identified by what color?
Purple
Iron (III) Oxide
Fe2O3
P3Cl10
Triphosphorus Decachloride
Lithium Nitride
Li3N
AuClO4
Gold (I) Perchlorate
What is the ground state electron configuration for Bromine?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
PbS2
Lead (IV) Sulfide
tetraphosphorus triselenide
P4Se3
Magnesium Phosphide
Mg3P2
Lead (II) Phosphate
Pb3(PO4)2
What is the noble gas configuration for Tungsten?