Number of Valence electrons in the 1st column
Lewis dot for Hydrogen
H with one dot
Draw the Lewis dot for Sodium
1 dot around Na
What subatomic particles does the Bohr Model Show?
All of them - protons, neutrons, and electrons
What do the rows (periods) in the periodic table show us?
How many shells/energy levels/rings an atom has.
Number of Valence electrons in the 2nd column
2
Lewis dot for O
6 dots around O
What is the Lewis Dot Structure for Aluminum
3 dots around Al
What element is this Bohr Model showing?
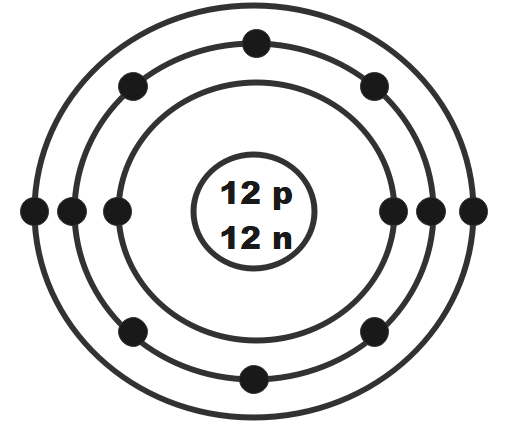
Magnesium
What does the column (group) number tell us on the periodic table?
The number of valence electrons, if more than 10, subtract 10
Number of Valence electrons in the 13th column
The Lewis Dot Structure of Boron.
3 dots around B
Draw the Lewis dot for Helium
2 dots around H
What element is this?
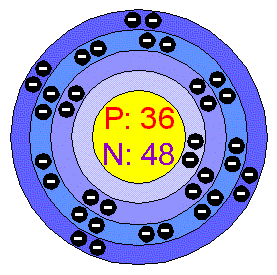
Krypton
What is the top number in an element's box called and what does it tell you (two things)

Atomic # - # of protons, also # of electrons
Number of Valence electrons in the 18th column (INCLUDE THE EXCEPTION).
8 except for He which has only 2
The Lewis Dot Structure for Chlorine (Cl).
7 dots around Cl
Lewis dot for Potassium
1 dot around K
How many electrons does Calcium (Ca) have?
20
What is the element's abbreviation in a element box called?

Chemical Symbol
Define a valence electron
Electrons on the outer shell, responsible for reactions
The Lewis Dot Structure of Selenium (Se).
6 dots around Se
8 dots around Xe
How many neutrons does Chlorine (Cl) have?
35 - 17 = 18
What is the bottom number in an element's box called and how do I use it to calculate number of neutrons?

Atomic mass, round it and subtract atomic number for number of protons.