What is the difference between the atomic structure of an element and of a compound?
What is elements are made up of one type of atom and compounds are made of two or more
A change in state of matter.
What is a physical change
What is the definition for density?
What is the amount of matter that occupies a certain amount of space
Some substances may have luster. What does the word "luster" mean?
What is to be shiny or reflect light
How are elements on the Periodic Table arranged?
What is by increasing atomic number
Is CO2 an element or compound?
What is a compound
Burning a candle.
What is a chemical change
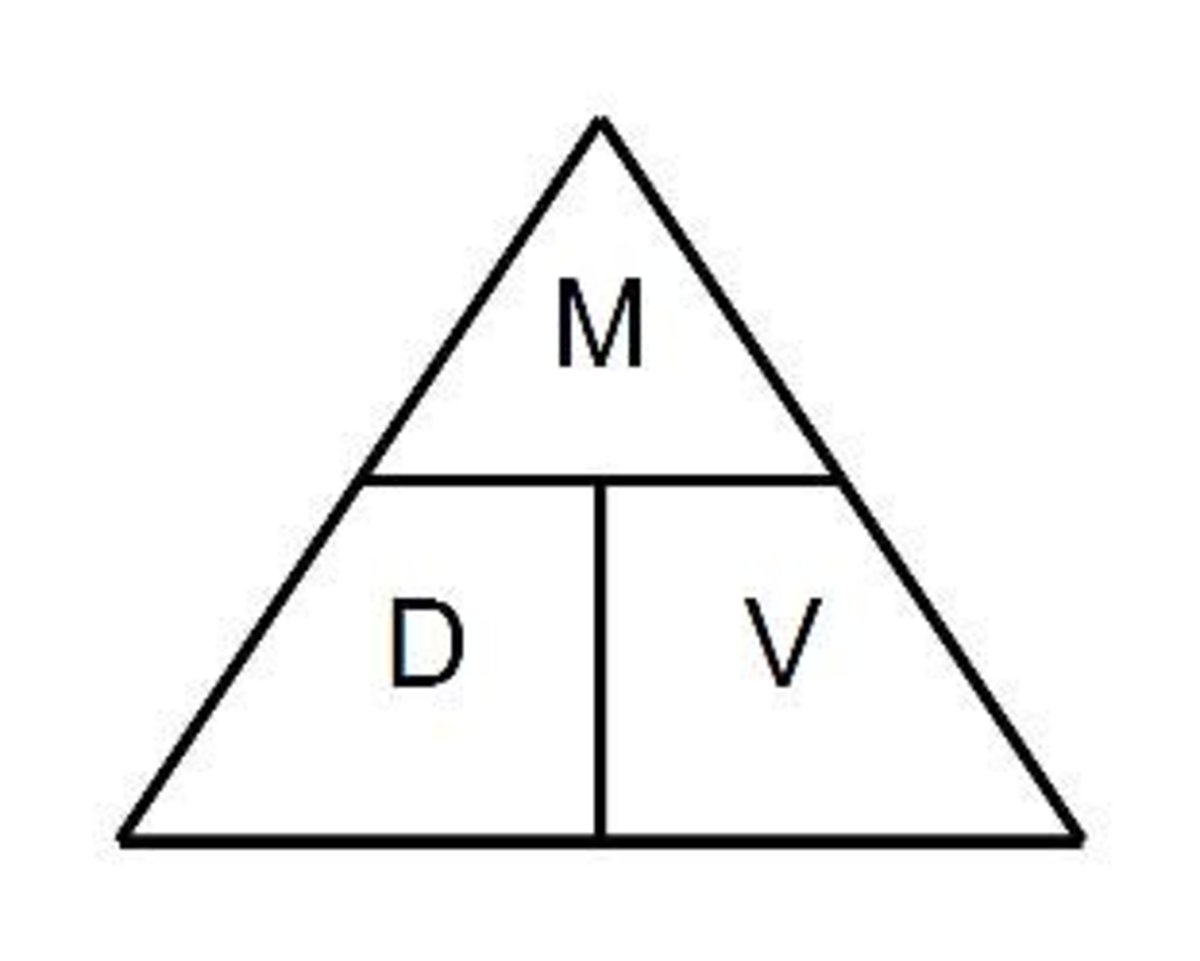
What is the formula to calculate density?
What is density = mass / volume
Which classification would contain elements that are brittle?
What is nonmetals
Define a group.
What is a vertical column of elements on the Periodic Table
In the chemical symbol FeO, how many elements make up this substance?
What is two elements
All chemical changes will --
What is create a new substance
Draw a density triangle on the board. Label each part of the triangle with the correct measurement.
What is another name for metalloids?
What is semi-metals
Define a row.
What is a horizontal line of elements on the periodic table
Given the picture below, find the number of protons, neutrons and electrons for Calcium.
![]()
What is 20 protons, 20 neutrons and 20 electrons
What is the difference between a chemical and physical change?
What is a chemical change creates a new substance and a physical change just changes the appearance of the substance
What is the formula to calculate volume?
What is volume = mass /density
What is the difference in density between metals and nonmetals?
What is metals have a high density and nonmetals have a low density
Define valence electrons.
What is electrons located in the outermost shell of an atom
Can a substance be an element and a compound at the same time? Explain your answer.
What is no because elements are made of one type of atom and compounds are not.
Alan is a lab mixing two chemicals. He notices as he mixes the chemicals, the solution starts to change colors and black specks begin to form at the bottom of the beaker. Those black specks can be best referred to as what?
What is a precipitate
Miles is observing a solid piece of gold with a mass of 250 grams. He has a beaker filled with 100 mL of water. When he add the piece of gold to the beaker, the water level rises to 150 mL. What is the density of the piece of gold?
What is 5 g/mL
Why can Polonium (Po) be classified as a metal instead of a metalloid?
What is because it has chemical reactivity similar to a metal
True or False: All elements have Bohr Models. (explain your answer)