Balancing
Balancing
changes
What does the Law of Conservation of Mass tell you? During a Chemical reaction Matter can’t be _____________or _________________
created or destroyed
What do you call the substances that react with each other in a chemical reaction?
Reactants
Atomic mass is calculated by
_____________________________________
Adding protons and neutrons.
When Marissa blows up a balloon what happens to the gas inside?
The air particles are spread out and fill up all of the space
3 ways to recognize a physical change
change in state of matter
no new substances created
easily reversed
Which of the pictures below illustrates that the law of Conservation says the products and reactants must be the same? Circle the correct answer.
the top one
What do you call the substance that comes out of a chemical reaction?
Product
The outside layer of electrons are called ___________
_________________________
Valence Electrons
Describe in detail what happens when you add heat to a solid.
The molecules/atoms start moving faster, spread out, and turn into a liquid (melt)
3 ways to recognize a chemical change
color change.
A new product was created.
light, sound, heat, or precipitate created.
not easily reversible.
is this equation balanced
show your work
Reactants - Iron - 3 Hydrogen 4 Oxygen 2
Products - Iron - 3 Hydrogen - 4 Oxygen -4
What are the reactants?
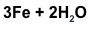
What type of elements are malleable, ductile, and brittle?
Metals
Define density
Density is a property of a substance that describes the relationship between the mass and volume of a substance. it is expressed as grams per ML, or grams per cm3
What happens when you mix vinegar and baking soda?
and how do you know?
Chemical reaction - a gas (CO2) is Created.
How many total atoms?
72

.What are the reactants
6CO2 - Carbon dioxide
6H2O - Water
When 2 atoms of the same element bonded together it's called ____________
Diatomic
If you had heat to a solid it turns to a _________and if you add heat you get a _______
liquid, gas
In a cup, you mix water and salt. What is the water? Solvent or Solute?
Solvent.
In a chemical reaction, the Mass of the Reactants is equal to __________________________________________________.
The mass of the products.
Balanced or not - Show your work 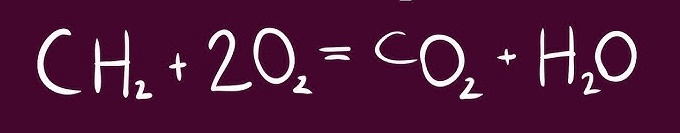
No
C - 1 C - 1
H - 2 H - 2
O - 4 O - 3
 What are these called
What are these called
Erlenmeyer flask
How does popcorn pop?
( use google )
As the kernel heats up, the water expands, building pressure against the hard starch surface. Eventually, this outer layer gives way, causing the popcorn to explode. As it explodes, the soft starch inside the popcorn becomes inflated and bursts, turning the kernel inside out.
In a cup, you mix water and salt. what is the salt? Solvent or Solute?
Solute.
Balanced or not - Show your work.

Nope 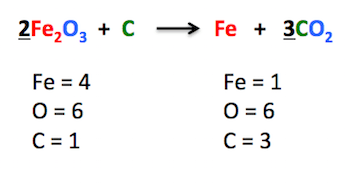
What are the products?
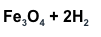
What lab tool do you use to measure mass?
Ballance.

What do each of the variables mean?
D - Density
M - Mass
V - Volume
What 2 things can cause a change in state of matter?