What is the range of electronegativity in covalent polar bonds?
What is 0.3 to 1.6?
What are lewis structures?
What is how valence electrons are arranged?
What's the molecular geometry of a molecular structure that has 4 bonded pairs and 0 non-bonded pairs?
What is tetrahedral?
What force do diatomics always have?
What is london dispersion force (LDF)?
What is within?
Metals and non-metals have what type of bond?
What are 2 electrons?
Linear, trigonal planar, and tetrahedral geometries all have what polarity?
What is non-polar?
What kind of bond do metal/nonmetals always have?
What intermolecular force has the lowest boiling point?
What is London dispersion forces (LDF)?
What is valence electrons are shared?
What does the octet rule say?
What is that atoms have 8 electrons in their valence shell?
What is the order from greatest to least force strength of intramolecular forces?
What is metallic bonding, Ionic bonds, hydrogen bonding, dipole-dipole, and London dispersion forces?
Which types of geometry have 2 bonded pairs?
What are bent and linear?
Non-polar covalent bonds are between what elements?
What are diatomic elements?
What are the exceptions to the octet rule?
What are hydrogen (needs 2 e-) and boron (needs 6 e-)?
What is the geometric shape of H2 and O2 ?
What is linear?
Dipole-Dipole is the attraction between what molecules?
What are polar molecules?
Ionic bonds/forces create what ions?
What are positive and negative ions?
HCl has what bond type?
What is polar?
What is missing in this lewis structure of NH4+ ? 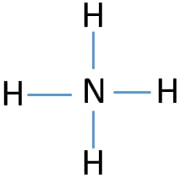
What are brackets and the charge?
What is the geometric shape of BF3 ?

What is trigonal pyramid?
What force is NCl3 (nitrogen and chlorine)?
What is Dipole- Dipole?
Hydrogen bonding is only seen when hydrogen is with what?
What is Florine, Oxygen, or Nitrogen?