It makes up everything around us including living and non-living materials.
What are atoms?
What are compound and elemental molecules?
It is how we can identify an element (hint: there are two)
What are physical and chemical properties?
It is the column (vertical) that places elements with similar characteristics together.
What are groups or families?
It is the a pure substance that cannot be broken down into other substances by chemical or physical means.
What are elements.
It is a subatomic particle that is found in the nucleus and is positively charged.
What is proton?
It is a type of molecule shown below
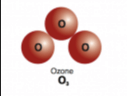
What is an elemental molecule?
It is a type of molecule that chemically bonds two or more of the SAME elements together.
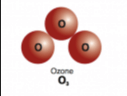
What is an elemental molecule?
It is the horizontal rows that represent the number of electrons in the valence electron shell.
What are periods?
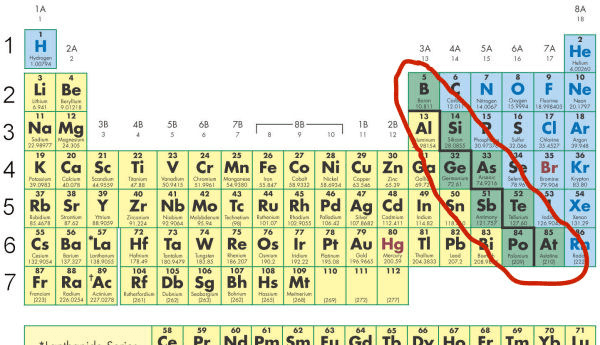
It is the section circled in the periodic table. Tell about its properties.
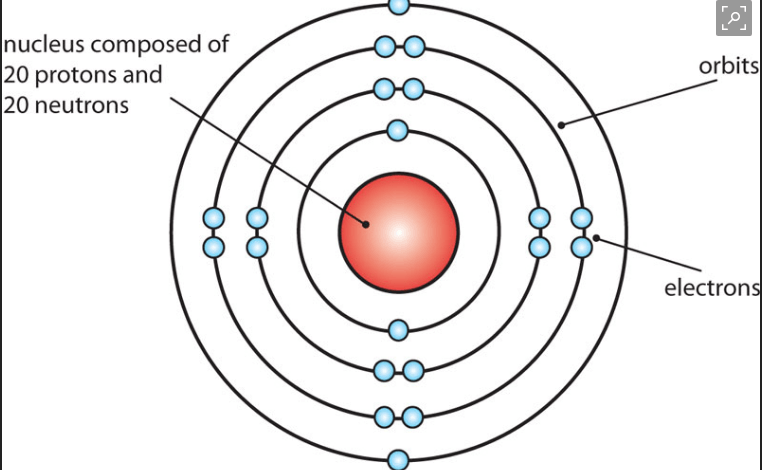
It is the subatomic particles inside of an atom (name the charges as well)
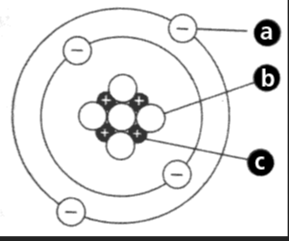
a- electrons (negative)
b- neutrons (equal/zero/neutral)
c-protons (positive)
It is synonym for a molecule with two elements chemically bonded.
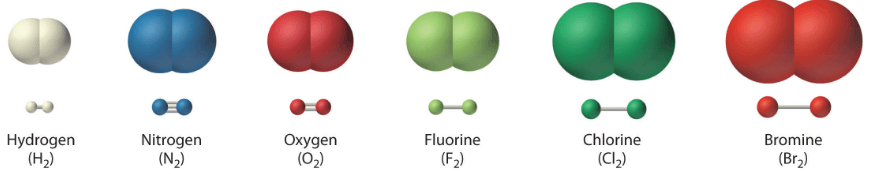
What is diatomic?
It is which type of property?

What is a physical property?
It is this element on the periodic table
What is calcium?
It is the group to the right of metalloids include their properties.
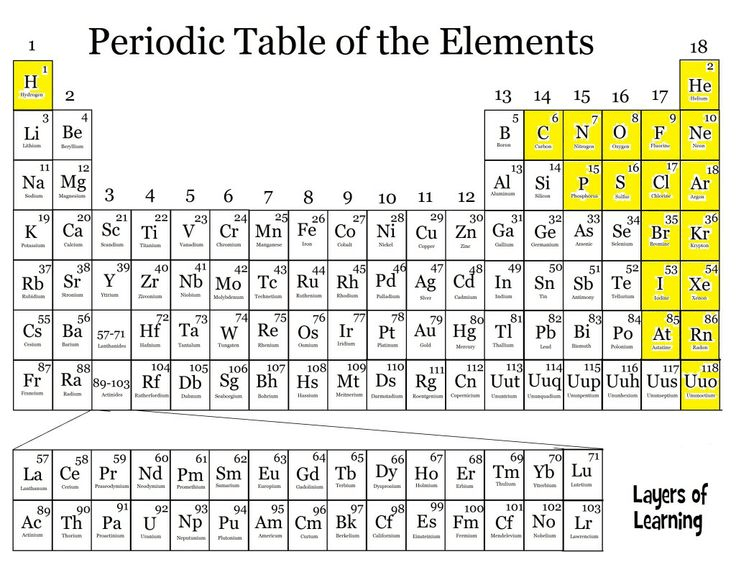
What are non-metals? They are not good conductors, low melting and boiling points, brittle
It is the function of each subatomic particle.
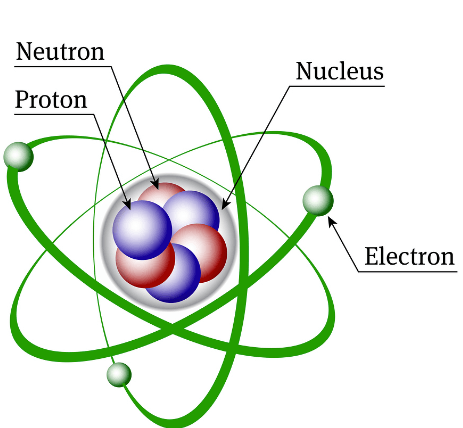
What is
nucleus- gives mass holds protons and neutrons
protons- positive energy level of element and attracted to electrons
electrons- orbit around nucleus determine energy level and attracted to protons
neutrons- stability and mass
Is the type of molecule H20, C02 and C6H1206
What is a compound molecule?
The are examples of chemical properties. Name at least four.
What are oxidation (rust), toxic, radioactivity, flammability, combustability
It is the patterns of which the periodic table is put together. Name at least four.
What are groups/families, periods, atomic number/protons/atomic mass, types of elements metals, metalloids and non-metals
It is the element found in group nine and period four.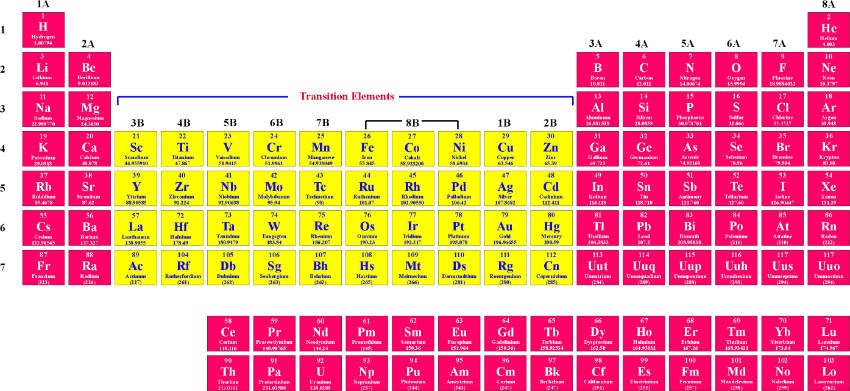
What is Mt (element #109)
It is how atomic mass is found in a stable element.
What is the number of protons and neutrons added together.
It is the similarities shared between a compound and elemental molecules. Name at least 4
.What are both are made of atoms, types of molecules, subatomic particles, elements, chemically bonded
It is the reason different periodic tables vary in the number of elements.
It is that new elements are being discovered.
It is the group and period number of Radium.
What is group 2 and period 7?