GFM
Which formula is an empirical formula?
- N2O4
- NH3
- C3H6
2. NH3
Which type of reaction occurs when a compound is separated into its elements?
Decomposition
TRUE OR FALSE: This is a Balance chemical equation:
C4H10 + Cl2 → C4H9Cl + HCl
TRUE
What is the percent composition by mass of nitrogen in the compound N2H4 (gram-formula mass = 32 g/mol)?
88%
Determine the mass of 25 moles of acetylene (gram-formula mass = 26 g/mol).
25 moles = x/ 26 = 650 g
What is the empirical formula for glucose?
CH2O
What type of equation is this:
2Al(s) + 3Cl2(g) → 2AlCl3(s)
synthesis
TRUE OR FALSE: This reaction occurs in water
NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
TRUE
What is the gram-formula mass of Fe(NO3)3?
242 g/mol
What is the number of moles of CO2 in a 220.-gram sample of CO2 (gram-formula mass = 44 g/mol)?
5.0 mol
What is the molecular formula for glucose?
C6H12O6
What type of equations is this:
2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)
Single Replacement
What is the main function of coefficients in balanced chemical equation?
Coefficients represent number of moles of each substance and help conserve mass and change in the chemical reaction.
What is the percent composition by mass of oxygen in Ca(NO3)2 (gram-formula mass = 164 g/mol)?
59%
DOUBLE JEOPARDY: Given the reaction: 2Al + 3H2SO4 → 3H2 + Al2(SO4)3
The total number of moles of H2SO4 needed to react completely with 5.0 moles of Al is
7.5 moles
The empirical formula for butene is...
CH2
What type of reaction is this:
AlCl3(aq) + 3KOH(aq) → Al(OH)3(s) + 3KCl(aq)
Double Replacement
DOUBLE JEOPARDY: Balance the following equation: P4(s) + Cl2(g) → PCl3(ℓ) + energy
1:6:4
What is the gram-formula mass of Ca(OH)2?
74 g/mol
DOUBLE JEOPARDY: Given the balanced equation for the reaction of butane and oxygen:
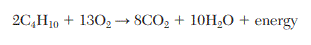
How many moles of carbon dioxide are produced when 5.0 moles of butane react completely?
20. mol
Which two terms represent types of chemical formulas?
empirical and molecular
During all chemical reactions, charge, mass and energy are
conserved
DOUBLE JEOPARDY Balance the following equation
___LiOH + ___CO2 → ___ Li2CO3 + ___H2O
2:1:1:1
Show a numerical setup for calculating the percent composition by mass of phosphorus in P4O10 (formula mass = 283.89 u).
((4 x 31)/283.89 ) x 100
Given the balanced equation representing a reaction:
Al2(SO4)3 + 6NaOH → 2Al(OH)3 + 3Na2SO4
The mole ratio of NaOH to Al(OH)3 is
3:1