There is more than one definition for the word "mole" True or False?
True
How many molecules do we have in a 2.00 mole sample of water?
1.20 x 1024 molecules
The word mole is only found in chemistry. True or False?
False
How many moles are in 4.8 x 1023 atoms of tungsten?
8.0 x 101 moles tungsten
What is Avogadro's number?
6.022 x 1023
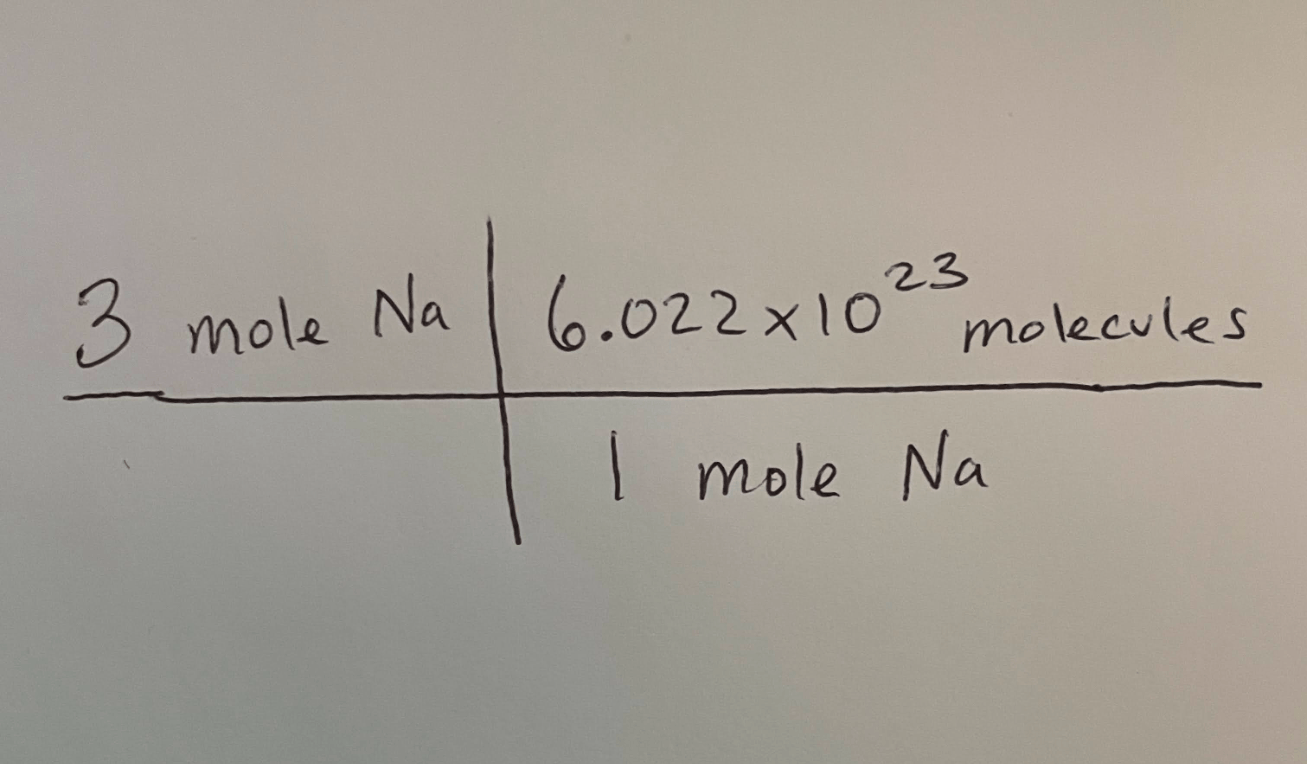
This is set up correctly. True or False?
True
How many particles are in one mole?
6.022 x 1023 particles
The mole is the SI Unit for the _________ of a chemical substance.
The amount
How many atoms are in a mole?
6.02 x 1023 atoms
The number of 'particles' in our lab was larger than the size of a mole. True or false?
False
Convert 9.02 x 1020 atoms of Iron to moles of Iron.
1.50 x 10-3 moles
How do we cancel out units?
Make sure we have one unit on the top and bottom of the equation
What are three common units for Avogadro's number?
Particles, Molecules, Atoms, Formula units, Ions
How many ions are in 0.053 moles of Fe+2?
3.2 x 1022 ions