Which type of equation is this: 2K + I2 ---> 2KI
a) Synthesis c. Decomposition
b) Single Replacement d. Double Replacement
Which type of equation is this: 2K + I2 ---> 2KI
a) Synthesis
Predict Product
Mg + O2 -->
2Mg + O2 --> 2MgO
___N2 + _____ H2 ---> ______NH3
a. 2, 2, 2 b. 1, 1, 2 c. 1, 3, 2 d. 1, 2, 2
_1_N2 + _3_ H2 ---> _2_NH3
c. 1, 3, 2
Based on the equation below, how many moles of hydrogen gas are produced from 8.80 moles of aluminum?
2Al + 6HCl ---> 2AlCl3 + 3H2
a. 5.47 moles
b. 13.2 moles
c. 26.4 moles
d. 1.5 moles
e. 2.0 moles
You set up a proportion
2 Moles Al for every 3 moles of H2
Therefore if we have 8.8 moles of Al we need to set up a proportion. 2/3 =8.8/n
2*n = 3*8.8
n= (3*8.8)/2
which is 13.2 mole Al
Reactant and Products are separated by the .....
Arrow (→ )
Which type of equation is this:
2KBr + Ca(NO3)2 ---> CaBr2 + 2KNO3
a) Synthesis c. Decomposition
b) Single Replacement d. Double Replacement
Which type of equation is this:
2KBr + Ca(NO3)2 ---> CaBr2 + 2KNO3
d. Double Replacement
Calcium bromide, CaBr2, reacts with sodium carbonate, Na2CO3, in a double displacement reaction to form:
CaBr2 + Na2CO3 -->________________
a. CaCO3 + 2 NaBr
c. Ca + CO2 + 2 NaBr
b.BrCO3 + CaNa
d. Ca2CO3 + NaBr2
Calcium bromide, CaBr2, reacts with sodium carbonate, Na2CO3, in a double displacement reaction to form:
CaBr2 + Na2CO3 --> CaCO3 + 2 NaBr
__CCl4 + __ H2O ---> __ CO2 + __ HCl
a) 0, 2, 0, 4 b) 2, 4, 2, 8
c) 1, 1, 1, 4 d) 1, 2, 1, 4
_1_CCl4 + _2_ H2O ---> _1_ CO2 + _4_ HCl
a) 0, 2, 0, 4
b) 2, 4, 2, 8
c) 1, 1, 1, 4
d) 1, 2, 1, 4
20. What does the law of conservation of mass mean?
a. No matter is ever destroyed or created.
b. Matter can only be created.
c. New matter is constantly being made.
d. All matter is constantly destroyed.
a) No matter is ever destroyed or created
Subscript tells ......
how many atoms of each element are present in a compound or molecule
Which of the following represents a decomposition reaction?
a. A + B ---> AB c. AB ---> A + B
b. A + B ---> C + D d. AB + CD ---> AD + CB
c. AB ---> A + B
Copper chloride and hydrogen sulfide react according to the equation below, which choice would give the balanced products of the reaction?
CuCl2 + H2S --> _________________
a) 2HCl + 2CuS b. 2HCl + CuS c. HCl + 2CuS d. CuCl2HS
You have to know the oxidation number of Cu2+ is S2- H+ and Cl- in order to actually answer these questions crisscross.
As well as balance the equation
b. 2HCl + CuS
__ Na2CO3 + __ HCl ---> __ NaCl + __ H2CO3
a) 1, 2, 2, 1 b) 2, 1, 1, 2
c) 2, 2, 3, 1 d) 2, 4, 1, 1
_1_ Na2CO3 + _2_ HCl ---> _2_ NaCl + _1_ H2CO3
a) 1, 2, 2, 1 b) 2, 1, 1, 2
c) 2, 2, 3, 1 d) 2, 4, 1, 1
18. A chemical reaction occurs in a beaker and takes 5 seconds to happen. The reaction is repeated, but this time the beaker is heated up. What will likely happen?
a. The reaction will take the same amount of time
b. The reaction will take longer than 5 seconds to happen
c. The reaction will happen in less than 5 seconds
d. There is no way to know what will happen.
c. Reaction would be less than 5 seconds because heat typically speeds chemicals reactions up
Coefficient
The coefficient tells you how many molecules of that substance there is or number of moles (amount)
Which balanced equation represents a single replacement reaction?
(a) Mg + 2AgNO3 --> Mg(NO3)2 + 2Ag
(b) 2Mg + O2 --> 2MgO
(c) MgCO3 --> MgO + CO2
(d) MgCl2 + 2AgNO3 --> 2AgCl + Mg(NO3)2
Which balanced equation represents a single replacement reaction?
(a) Mg + 2AgNO3 --> Mg(NO3)2 + 2Ag
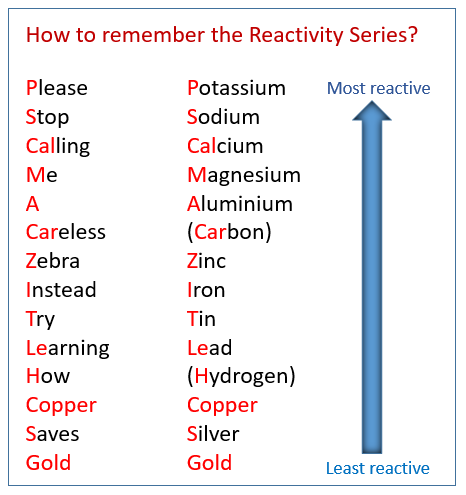 A student mixed solid aluminum into a solution of magnesium chloride. Which of the following would be the result?
A student mixed solid aluminum into a solution of magnesium chloride. Which of the following would be the result?
a) Option 1: Al + MgCl2 --> Mg + AlCl2
b) Option 2: Al + MgCl2 --> No reaction
A student mixed solid aluminum into a solution of magnesium chloride. Which of the following would be the result?
b) Option 2: Al + MgCl2 --> No reaction
Which of the following correctly balances this equation?
a. 2NaOH + 2CuCl2 ---> 2Cu(OH)2 + 2 NaCl
b. 2NaOH + CuCl2 ---> Cu(OH)2 + NaCl
c. NaOH + CuCl2 ---> Cu(OH)2 + 2 NaCl
d. 2NaOH + CuCl2 ---> Cu(OH)2 + 2 NaCl
Which of the following correctly balances this equation?
d. 2NaOH + CuCl2 ---> Cu(OH)2 + 2 NaCl
Balance the following equation to demonstrate the conservation of atoms in a reaction. Choose the answer which provides the correct coefficients for each reactant and product.
____ CS2 + ____ O2 → ____ CO2 + ____ SO2
a. (1, 3, 1, 2) b. (1, 2, 1, 1)
c. (1, 6, 1, 2) d. (2, 6, 2, 2)
____ CS2 + ____ O2 → ____ CO2 + ____ SO2
a. (1, 3, 1, 2)
Another word for coefficient in chemistry is called :
Moles
The balanced chemical equation below represents a reaction between glucose (C6H12O6) and oxygen (O2). C6H12O6 + O2 ---> 6 CO2 + 6 H2O
Which type of reaction does this equation represent?
a. single displacement c. decomposition
b. Synthesis d. Combustion
d) Combustion
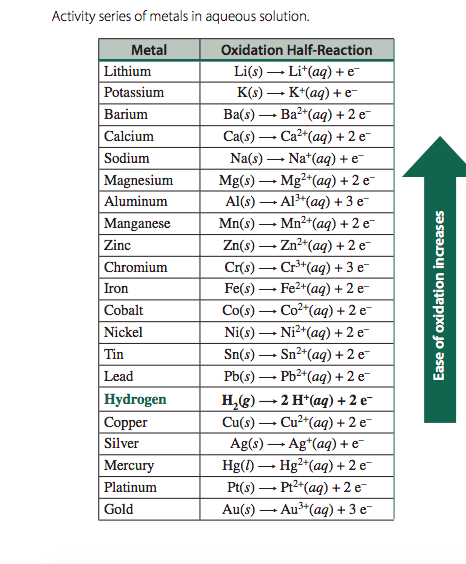
A student poured 25 ml of magnesium hydroxide (Mg(OH)2) into a beaker. Then, he added 8 grams each of aluminum (Al), lithium (Li), iron (Fe) and copper (Cu). Use the table to determine the product of this reaction.
a) Mg + Al(OH)3 b) Mg + LiOH
c) Mg + Fe(OH)2 d) Mg + Cu(OH)2
b) Mg + LiOH
Strongest/Most Reactive metal out of the options strong enough to take OH from Mg.
Hydrazine, N2H4, and dinitrogen tetroxide, N2O4, react to form gaseous nitrogen (N2) and water. Which of these represents a properly balanced equation for this reaction?
a. N2H4 + N2O4 ---> N2 + H2O
b. 2 N2H4 + N2O4 ---> 3 N2 + 4 H2O
c. N2H4 + N2O4 ---> N + H2O
d. 6N + 4 H2O ---> N2H4 + 2 N2O4
Hydrazine, N2H4, and dinitrogen tetroxide, N2O4, react to form gaseous nitrogen (N2) and water. Which of these represents a properly balanced equation for this reaction?
b. 2 N2H4 + N2O4 ---> 3 N2 + 4 H2O
19. If a chemical reaction occurs that starts with 5 hydrogen atoms, what must be true about the products?
a. The product must have 5 hydrogen atoms.
b. The product must have less than or equal to 5 hydrogen atoms.
c. The product must have more than or equal to 5 hydrogen atoms.
d. There will be no hydrogen atoms in the product.
c. The product must have more than 5 hydrogen atoms.
Hydrogen is elemental and they typically role in two therefore when balancing chemical equations we may need more Hydrogens
Steps for balancing equation
Count number of each element
Deal with one element at a time
Change Coefficient till balanced not subscript