2H2S(g) + SO2(g) ⇌ 3S(s) + 2H2O(g)
At 298 K, the standard enthalpy change, ∆Ho, for the reaction represented above is -145 kilojoules.
Predict the sign of the standard entropy change, ∆So, for the reaction.
Explain the basis for your prediction.
Statement that ΔS0 is negative
3 moles of gas → 2 moles of gas plus solid
2 H2(g) + O2(g) → 2 H2O(g)
For the reaction represented above at 25°C, what are the signs of ΔH°, ΔS°, and ΔG°?
All negative
2 Al(s) + 3 Zn2+(aq) → 2 Al3+(aq) + 3 Zn(s)
Write the complete electron configuration for Zn2+
1s22s22p63s23p63d10
3 Ag+(aq) + M(s) → 3 Ag(s) + M3+(aq) E° = +2.46 V Ag+(aq) + e- → Ag(s) E° = +0.80 V
According to the information above, what is the standard reduction potential for the half-reaction
M3+(aq) + 3 e- → M(s)
-1.66 V
What thermodynamic property would be zero for a pure, perfect crystal at 0 K
Entropy
When solid ammonium chloride, NH4Cl(s), is added to water at 25°C, it dissolves and the temperature of the solution decreases. Which would be the values for the ΔH and ΔS for the dissolving process?
both (+)
H2O2(aq) + OCl—(aq) ⇋ O2(g) + Cl—(aq) + H2O(l)
For the reaction represented above, ΔG°298 is −197 kJ/ molrxn and the value of ΔS°298 is 144 J/(K· molrxn)
Calculate the value of the equilibrium constant, K, for the reaction at 298 K
3 x 1034
For the reaction represented opposite at 25°C,
2H2(g) + O2(g) → 2H2O(g)
what are the signs of ΔH°, ΔS°, and ΔG°?
All (-)
2 NO(g) + O2(g) ⇋ 2 NO2(g) ΔH° = -112 kJ /molrxn
Answer the following questions about the reaction represented above at 298K.
Absolute Entropy at 298K (J / (K.mol))
NO(g) 211
O2(g) 205
NO2(g) 240
- 68 kJ mol-1 (favorable)
2H2O2(aq) → 2 H2O(l) + O2(g) ΔH° = −196 kJ/molrxn
The decomposition of H2O2(aq) is represented by the equation above. The reaction is thermodynamically favorable. The signs of ΔG° and ΔS° for the reaction are what?
(-) Gibbs
(+) Entropy
Calculate the quantity of electricity (Coulombs) necessary to deposit 100.0 g of copper from a CuSO4 solution.
3.037 x 105 C
Write equations for the half–reactions and the overall cell reaction, and calculate E°cell for each of the voltaic cells diagrammed below.
Pt | I2(s) | I–(aq) || Cl–(aq) | Cl2(g) | Pt
Overall: Cl2(g) + 2 I–(aq) → I2(s) + 2 Cl–(aq)
Ecell = +0.823 V
How many hours will it take to plate out copper in 200.0 mL of a 0.1500 M Cu2+ solution using a current of 0.200 amp? (1 A = 1 C/s)
8.04 hrs.
Overall reaction: Pb(s) + PbO2(s) + 2H+ (aq) + 2HSO4−(aq) →2PbSO4(s) + 2H2O(l)
Cathode half-cell reaction: PbO2(s) + 3H+(aq) + HSO4−(aq) + 2e-→ PbSO4(s) + 2 H2O(l)
The equations above represent reactions associated with the operation of a lead storage battery. The first is the overall reaction that occurs as the battery produces an electrical current, and the second is the half-reaction that occurs at the cathode.
Write the equation for the half-reaction that occurs at the anode as the battery operates.
Pb(s) + HSO4-(aq) → PbSO4(s) + H+(aq) + 2e-
What current is needed to deposit 0.480 g of chromium metal from a solution of Cr3+ in a period of 1.50 hr?
0.495 A or C/s
Calculate the solubility product of AgI at 25.0 °C, given the following data:
Reduction half-reaction E° (V)
AgI(s) + e¯ ---> Ag(s) + I¯ -0.15
I2(s) + 2e¯ ---> 2I¯ -0.54
Ag+ + e¯ ---> Ag(s) +0.80
8.51 x 10-17
Determine the values of E°cell and ΔG° for the following reactions.
Al(s) + 3 Ag+(aq) → Al3+(aq) + 3 Ag(s)
2.476 V
–716.7 kJ
A voltaic cell is constructed to carry out the reaction,
2Cr2+(aq) + Pb2+(aq) → 2Cr3+(aq) + Pb(s)
If the [Cr3+] = 0.0300 M, [Pb2+] = 0.150 M and the [Cr2+] = 0.250 M, calculate Ecell at 25 ˚C.
You are provided only one half-reactions with its potential below:
2Cr2+(aq) → 2Cr3+(aq) + 2e– +0.408 v
+.312 V
Using the following reduction potentials, calculate the solubility product for AgCN at 298 K:
Ag+ + e¯ ---> Ag E = 0.80 V
AgCN + e¯ ---> Ag + CN¯ E = -0.01 V
2.0 x 10-14
Determine the values of E°cell and ΔG° for the following reactions.
4 IO3–(aq) + 4 H+(aq) →2 I2(s) + 2H2O(l) + 5 O2(g)
–0.03 V
+60 kJ
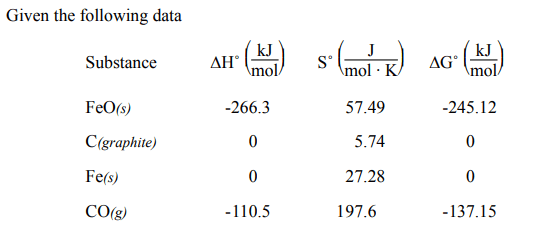
Calculate ∆G˚, ∆S˚, and ∆H˚ for the reaction,
FeO(s) + C(graphite) → Fe(s) + CO(g)
964 K
Balance the redox reaction below that is in basic solution:
O2 + Cr3+ --> H2O2 + Cr2O72-
8 OH- + 2Cr3++3O2 --> 3H2O2 + Cr2O72- + H2O
A voltaic cell consists of an anode compartment with a nickel electrode immersed in a NiSO4 solution and a cathode compartment with a copper electrode immersed in a CuSO4 solution. A salt bridge connects the two cells.
A current of 1.50 amps is observed to flow for a period of 2.00 hours. How much charge passes through the circuit during this time? How many moles of electrons is this charge equivalent to? (1 A = 1 C/s)
10800 C
0.112 mole e-
The following cell is maintained at 25°C. One half-cell consists of a chlorine/chloride, Cl2/Cl-, electrode with the partial pressure of Cl2= 0.100 atm and [Cl-]= 0.100 M. The other half-cell involves the MnO4-/Mn2+ couple in acidic solution with [MnO4-] = 0.100 M, [Mn2+] = 0.100 M, and [H+] = 0.100 M. Apply the Nernst equation to the overall cell reaction to determine the cell potential for this cell.
MnO4- + 8H+ + 5e- →Mn2+ + 4H2O E° = 1.507 V
Cl2 + 2e-→ 2Cl- E° = 1.360 V
0.017 V
Write equations for the half–reactions and the overall cell reaction, and calculate E°cell for each of the voltaic cells diagrammed below.
Pt | PbO2(s) | Pb2+(aq), H+(aq) || S2O82-(aq),SO42(aq) | Pt
Pb2+(aq) + 2H2O(l) + S2O82–(aq)→PbO2(s)+4H+ (aq)+ 2SO42–(aq)
+0.56 V