Which mixture could be separated using a magnet?
A. Pebbles and marbles
B. sand and seashells
C. Pepper and sugar
D. Iron filings and pepper
D.Iron filling and pepper
Does NOT allow (stops) heat or electricity from flowing through
Insulator

Particles get so small they cannot be seen
Dissolve

Anything that has mass and takes up space
Matter

Which of these would be a good conductor of electricity?
a. Cotton string
b. Copper wire
c. glass rod
d. Plastic tube
B. Copper Wire

The diagram shows a ball in a beaker filled with water. Which statement best describes the relationship of the ball and the water?
The water is less dense than the ball.
Allows (can) heat or electricity to flow through
Conductor

Ability to dissolve in water
Solubility

Amount of matter in an object (measured in kg or g)
Mass
A bottle of salad dressing might contain oil and water. Which ingredient has the least amount of density?
Oil
Which of the following is true about a plastic handle on a curling iron?
A. insulates heat
b. Soluble in water
C. magnetic

A. Insulates Heat
The boiling point of water
100 degrees Celsius

Why would you wear gloves when working with boiling water and a pan? Metal is a good ___________.
Thermal Energy

Appearances of an object: mass, magnetism, physical state, relative density, solubility, insulator/conductor
Physical Properties
A group of students are observing water being heated on a hot plate. They observe that the temperature is 53 degrees Celsius. How many more degrees must it rise before it boils?
47 degrees
Some students boiled water for awhile, until nothing was left in the pan. The water evaporated because it reached a temperature of –
a. 0 degrees Celsius
B. 100 degrees Celsius

B. 100 degrees Celsius
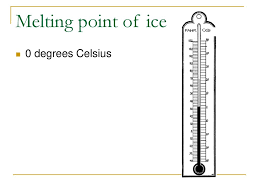
The freezing and melting point of water
0 degrees Celsius

What happens to the size of salt grains in water?
They get smaller until you can’t see them (dissolve)

Objects more dense sink in water; less dense objects float in water

Relative Density
If you melted an ice cube with a mass of 80 grams and an ice cube with a mass of 40 grams, would they melt at the same temperature?
YES! (melting point 0 degrees Celsius)
If powder is mixed with water until it’s no longer seen, what is being demonstrated?
A. Solubility
B. Density
C. Conductivity
D. mass
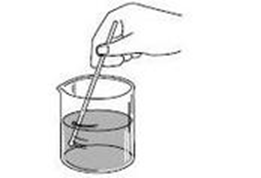
A. Solubility
4 jars were wrapped in various materials, and filled with water that is 88 degrees Celsius. After 10 minutes, students test the temperature again. Which property are the students testing?
A. Color, size, or shape
B. Magnetism
C. Thermal energy insulation
D. Solubility
Thermal Energy Insulation
Foam cups would float on water because they would be less dense than water. True/False
True

Ability of a solid to dissolve in a liquid

Solubility
A sidewalk was created with a mixture of items, including large chunks of salt. After it dried, it began to rain and later there were large holes in the concrete. Why?
The salt dissolved