Graphs of the data from laboratory investigations are used to
a) observe general trends and see the data in a more organized way
b) make observed data more accurate
c) prevent errors in measuring data
d) help change the original data tables
What is...
a) observe general trends and see the data in a more organized way
Four materials are put into small containers. These materials are then moved from the small containers into larger containers. Which material will spread out to completely fill a large container?
a) air
b) ice
c) sand
d) water
What is...
a) air
Use the periodic table below to answer the question. Potassium (K), atomic number 19, will most likely react with which of the following elements?
a) Sodium (Na), because it is in the same column.
b) Calcium (Ca), because it is in the same row.
c) Chlorine (Cl), because it is a nonmetal
d) Argon (Ar), because it is a noble gas.
What is...
c) Chlorine (Cl), because it is a nonmetal
In a parallel circuit, what will happen to Bulb 1 if Bulb 2 burns out?
a) Bulb 1 will also burn out.
b) Bulb 1 will flash on and off.
c) Bulb 1 will stay lit and stay the same brightness.
d) Bulb 1 will stay lit but will become less bright.
What is...
c) Bulb 1 will stay lit and stay the same brightness.
Ms. Dungee is testing the pH of her pool. She uses pH strips and it turns out her pool has a pH of 10. Correctly describe her pool.
a. the pool is acidic
b. the pool is neutral
c. the pool is basic/alkaline
d. the pool is dirty
What is...
c. the pool is basic/alkaline
Repeating experiments improves the likelihood of accurate results because the overall results are
a) less likely to prove the hypothesis correct.
b) more likely to prove the hypothesis correct.
c) less likely to be correct due to fewer errors being made.
d) more likely to be correct due to fewer errors being made.
What is...
d) more likely to be correct due to fewer errors being made.
Which of the following correctly pairs a phase of matter with its description?
a) Solid: Particles have no motion.
b) Liquid: Particles expand to fill any container in which they are placed
c) Gas: Particles have higher amounts of energy than when in the liquid phase.
d) Liquid: Particles are more strongly attached to one another than when in the solid phase.
What is...
c) Gas: Particles have higher amounts of energy than when in the liquid phase.
The atomic number of iron is 26, and the atomic mass is 55.847. What do these numbers mean in regard to protons, electrons, and neutrons?
a) There are 13 each of protons and neutrons, and the rest of the mass is the result of electrons.
b) There are 26 protons and 26 electrons. Some atoms of iron have 30 neutrons.
c) There are 26 protons and neutrons. Each particle has an atomic mass of 1.
d) There are 26 protons and 26 neutrons. Since neutrons have slightly more than protons, the mass is greater than 52.
What is...
b) There are 26 protons and 26 electrons. Some atoms of iron have 30 neutrons.
In cars with electric locks, electromagnets allow the doors to be locked and unlocked at the push of a button. Why is an electromagnet used for this kind of door lock?
a) Using electromagnets conserves electricity.
b) Using electromagnets prevents static shock.
c) Electromagnets can be easily turned off.
d) Electromagnets can attract metals or nonmetals.
What is...
c) Electromagnets can be easily turned off.
Sodium (Na) is a metal, and chlorine (Cl) is a nonmetal metal. If these two elements combine, what type of bond will form?
a. ionic bond
b. metallic bond
c. covalent bond
d. mixture bond
What is...
a. ionic bond
Which piece of equiment could be used to determine the mass of an earthworm?
a) triple beam balance
b) meter stick
c) graduated cylinder
d) beaker
What is...
a) triple beam balance
Which of the following is the best example of a pure substance?
a) peanuts
b) gold
c) milk
d) air
What is...
b) gold
What do the elements in group 4 have in common?
a) They are metals.
b) They are in the same period.
c) They have the same number of electrons.
d) They have 4 electrons on the outer shell.
What is...
d) They have 4 electrons on the outer shell.
Which of the following is an advantage of an electronic circuit over an electric circuit?
a) providing transfer of data
b) carrying electric current
c) creating a magnetic field
d) producing light
What is...
a) providing transfer of data
How many mL are in the graduated cylinder?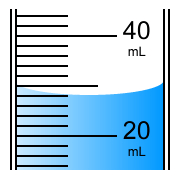 a. 25 mL
a. 25 mL
b. 28 mL
c. 24 mL
d. 30 mL
What is 28 mL?
A student was trying to determine which type of bee trap was best for collecting different types of bees. Trap A is the standard trap that was bought at the store. Trap B was made by the student to be larger. Trap C was made by the student to be smaller. The graph below shows how many of each bee type were collected in traps A, B, and C.
Based on the information provided, what is the student's control group?
What is...
Trap A
(it is the standard/the normal group with NO change)
Which statement correctly describes both gases and liquids?
a) Their shape changes when they are in a different container.
b) Their shape stays the same in any container.
c) Their volumes stay the same in any container.
d) Their volume changes when they are in different containers.
What is...
a) Their shape changes when they are in a different container.
Which characteristic is different in each isotope?
a) the position in the periodic table of the elements
b) the net charge of the nucleus
c) the mass of the protons in the nucleus
d) the number of neutrons in the nucleus
What is...
d) the number of neutrons in the nucleus
The diagram below shows a simple electrical circuit.
Which of the following would always increase the flow of current through the lights in the circuit shown above?
a) Decreasing the battery voltage and decreasing the resistance of the lights.
b) Increasing the battery voltage and increasing the resistance of the lights.
c) Decreasing the battery voltage and increasing the resistance of the lights.
d) Increasing the battery voltage and decreasing the resistance of the lights.
What is...
d) Increasing the battery voltage and decreasing the resistance of the lights.
Look at the beakers. Which of the following answers choices are correct?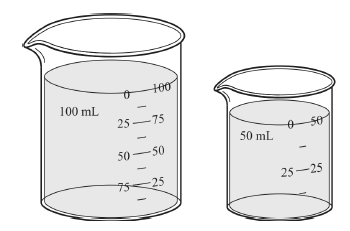
a) both beakers have the same mass
b) both beakers have the same volume
c) there is no difference
d) both beakers have the same density
What is...
d) both beakers have the same density
The number of meadow mice in a certain grassy field was determined each year from 1977 - 1989. The results are represented in the data table below.
During which time period did the greatest change in the size of the population of meadow mice take place?
a) 1977 - 1978
b) 1978 - 1979
c) 1984 - 1985
d) 1986 - 1987
What is...
b) 1978 - 1979
Steel is a metal that is made from iron and carbon. During the steel making process, iron and carbon are melted, blended together, and then allowed to harden into a solid. The iron and carbon do not chemically react with each other. After steel was made, 20 samples were taken from one piece and tested. Each sample contained 98% iron and 2% carbon.
Which of the following best describes steel?
a) element
b) compound
c) homogeneous mixture
d) heterogeneous mixture
What is...
c) homogeneous mixture
Copper (Cu) reacts with oxygen (O) to form copper oxide (CuO). The properties of CuO are most likely
a) different from copper or oxygen.
b) similar to both copper and oxygen.
c) similar only to copper.
d) similar only to oxygen.
What is...
a) different from copper or oxygen.
Students in a lab measure a current flowing through a long loop of wire.
If there is no battery connected to the wire, which of the following explains the source of the current?
a) The ammeter is acting as a current source.
b) There is a magnetic field inside the loop.
c) There is a fixed current running in a separate wire.
d) There is a static charge built up in the wire
What is...
b) There is a magnetic field inside the loop.
Emma is hungry and wants to make a smoothie. She adds strawberries, bananas, blueberries, and almond milk. She blends it all and enjoys her smoothie. Which of the following correctly describes Emma's smoothie?
a. element
b. mixture
c. atom
d. compound
What is...
b. mixture