What words must be included in your hypothesis?
IF, THEN, BECAUSE!!!
What happens to the kinetic energy of particles as temperature increases?
The kinetic energy of particles increases.
What charge does an electron have?
Negative
What is mass number?
# protons + # neutrons
List the seven colors of the rainbow, starting with "red".
Red
Orange
Yellow
Green
Blue
Indigo
Violet
What is the difference between a group and a period?
A group is a vertical column on the periodic table and a period is horizontal row on the periodic table.
What is the name of a positive ion? Of a negative ion?
Cation is positive and anion is negative.
What is the 2-8-8 rule?
The first energy level holds a maximum of two electrons and all other energy levels hold a maximum of eight electrons.
What is an independent variable?
The variable that is changed in an experiment.
What relationship do the variables in Avogadro's law have?
Number of particles and volume have a direct relationship.
Which subatomic particle has a charge of +1?
Proton
Identify the atomic number and the mass number in the isotope below.
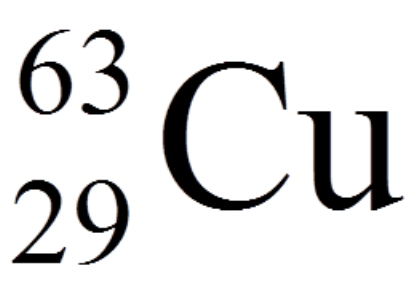
atomic # = 29
mass # = 63
What are the red arrows measuring?
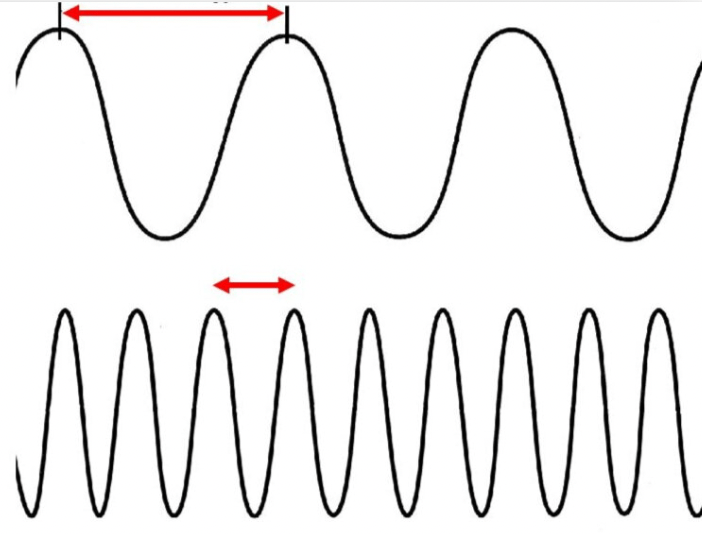
Wavelength
Would you classify the elements in pink as metals, nonmetals, or metalloids?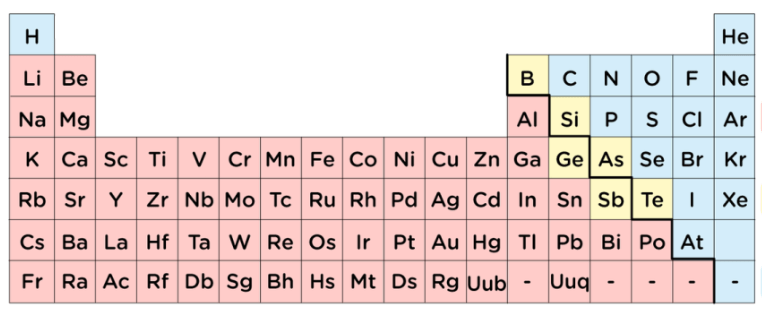
Metals
What is a valence electron?
A valence electron is an electron in the outermost energy level of an atom.
What type(s) of element(s) (metals, nonmetals, metalloids) come together to form ionic compounds?
What is a dependent variable?
What relationship do the variables in Charles' law have?
Temperature and volume have a direct relationship.
What charge does a neutron have?
Neutral/no charge
Identify the atomic number and the mass number in the isotope below.
silver-107
atomic # = 47
mass # = 107
Which wave has a higher frequency? (Assume the top wave is "a" and the bottom wave is "b".)

Would you classify the elements in blue as metals, nonmetals, or metalloids?
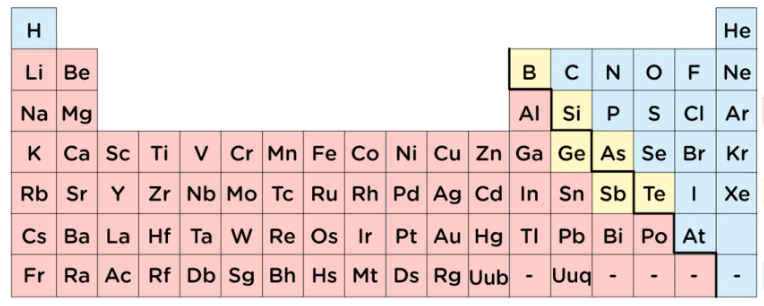
Nonmetals
How many valence electrons does an atom of nitrogen have?
5 valence electrons
What happens to the valence electrons in a metal and nonmetal when ionic compounds are formed?
Valence electrons are transferred from the metal to the nonmetal.
Does colored water evaporate faster than clear water?
In the experiment above, which type of variable would the time it takes the water to evaporate be?
Dependent variable
What relationship do the variables in Boyle's law have?
Pressure and volume have an indirect relationship.
Where are electrons, neutrons, and protons located within the atom?
electrons = energy levels outside of the nucleus
protons & neutrons = nucleus
How many neutrons are in bromine-80?
45
What form of light are human eyes able to detect?
Visible light
Would you classify the elements in yellow as metals, nonmetals, or metalloids?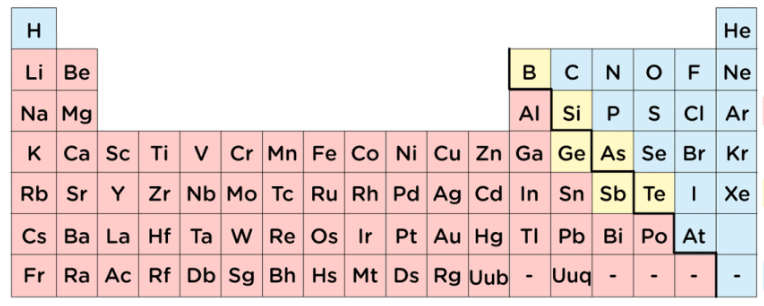
Metalloids
What is the charge of an atom with 33 protons and 33 electrons?
Neutral/no charge.
Illustrate the Lewis electron dot diagram of phosphorus (P).

Does the type of shoes a person wears affect how fast they can run?
What is the dependent variable?
The dependent variable is how fast the person runs.
How is kinetic energy different in the solid state, liquid state, and gaseous state?
In the solid state, the movement of particles is limited. In a liquid state, particles move past each other. In a gaseous state, particles have a significant amount of motion and move freely.
What is the name of the element with 29 protons?
copper (Cu)
Write the following in isotope notation:
protons = 32
neutrons = 40
electrons = 32
germanium-72
72/32 Ge
What is the difference between the ground state and the excited state of an atom?
In the ground state, the electrons are in their normal energy levels at a low-energy configuration. In the excited state, electrons are temporarily in higher energy levels.
What is the name of the group highlighted in bright yellow (group 18)?

Noble gases
What is the charge of an atom with 84 protons and 87 electrons?
-3
Determine the ionic compound that forms between strontium and nitrogen ions using the criss-cross method.
Sr3N2
Use the provided independent and dependent variables to create a hypothesis.
Independent variable: type of paper towels
Dependent variable: ability to absorb liquid
If given two different types of paper towels, then the paper towel that is thicker will absorb more liquid, because...
The volume of a kickball decreases when it is left outside overnight. What can you assume about the temperature at night?
The temperature decreased at night.
What is the chemical symbol of the atom with 37 protons?
Rb
In this picture, what number does "A" symbolize? What number does "Z" symbolize?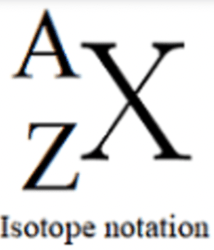
A = mass #
Z = atomic #
Which subatomic particle (proton, electron, neutron) is responsible for producing electromagnetic waves?
The movement of electrons is responsible for producing electromagnetic waves.
What is the name of the group highlighted in red (group 1)?

Alkali metals
Complete the table below.
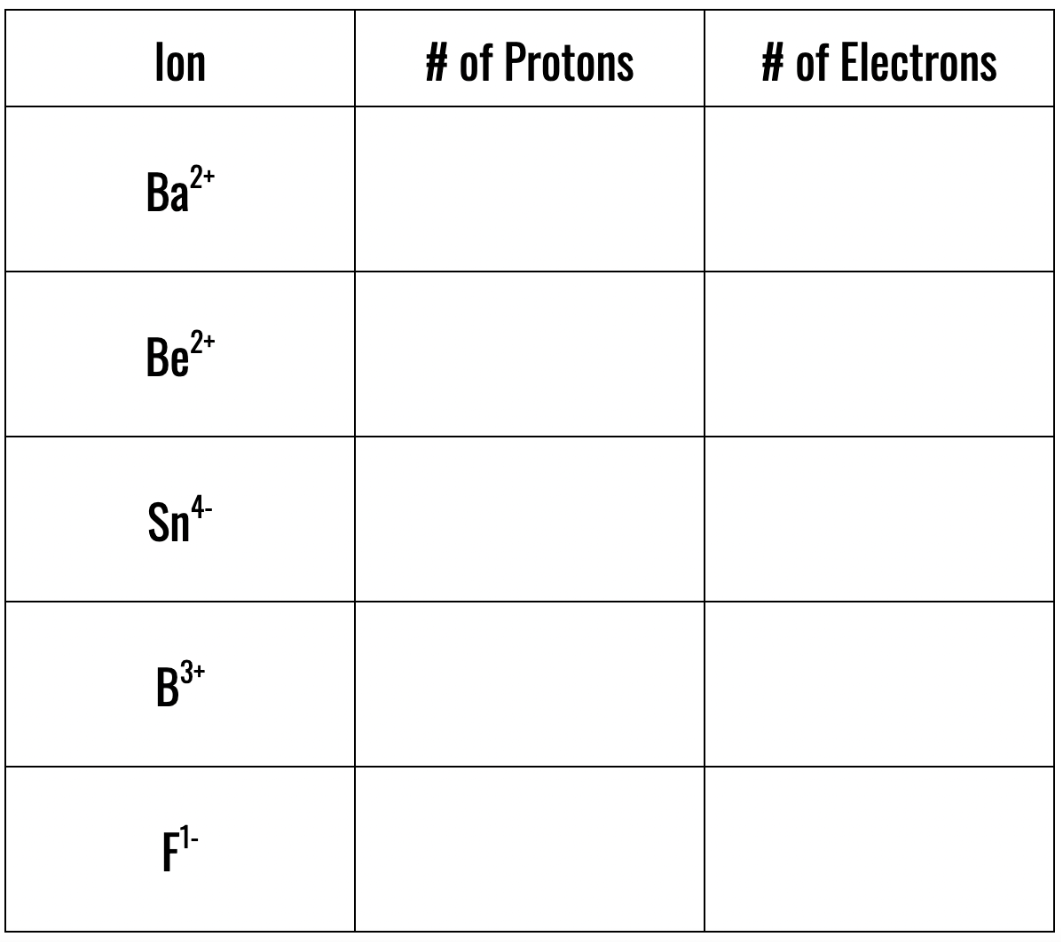
56 | 54
4 | 3
50 | 54
5 | 2
9 | 10
Determine the ionic compound that forms between rubidium and chlorine ions using the criss-cross method.
RbCl
A student wanted to see if their score on spelling tests would improve if they spent more minutes studying the spelling words.
What would the independent variable in this experiment be?
The independent variable is the amount of time spent studying for spelling.
You're helping to prepare the decorations for your cousin's birthday party. Describe what is happening to the number of particles and volume as you inflate each balloon.
Volume increases as number of particles increases.
How many protons does an atom with an atomic weight of 118.710 amu have?
50 protons
What is the same about isotopes of the same element? What is different about isotopes of the same element?
The # of protons stays the same. The # of neutrons changes.
The energy of a wave of yellow light and a wave of red light are measured. Which wave has the lower energy and why?
Red light has the lower energy because it has longer wavelengths.
What is the name of the group highlighted in orange (group 2)? 
Alkaline earth metals
Write the ion symbol for the ion with 79 protons and 78 electrons.
What is the overall charge of an ionic compound?
Zero (0)
Does a skateboard travel further on grass, the sidewalk, or on the paved road?
What is the independent variable?
Types of surfaces
What happens to the kinetic energy of water particles during the evaporation process?
The kinetic energy of water particles increases as water goes from a liquid state to a gaseous state.
How many electrons does a neutral atom of sulfur have?
16
Phosphorus-33 and phosphorus-30 occur in natural. The average atomic weight of phosphorus is 30.973 amu. Which isotope is most abundant in nature and why?
Phosphorus-30 is most abundant in nature, since the average atomic weight (30.973 amu) is closest to this value.
What is the difference between absorption and emission of energy?
absorption = energy is absorbed; electron moves up
emission = energy is released; electron moves down
What is the name of the group highlighted in yellow (group 17)?

Halogens
Will fluorine gain or lose electrons? Draw an energy level model of a neutral atom of fluorine and an energy level model of the ion that fluorine is most likely to form.
Fluorine gains electrons. 7 VE to start, 8 VE once an ion forms.
Illustrate the ionic bond formation of calcium sulfide (Ca2S2) using Lewis electron dot diagrams.
Ca + S --> Ca2+[S]2-
How will batting practice affect a baseball player’s batting average?
Create a hypothesis from this question.
If a baseball player increases the amount of time at batting practice, then their batting average will increase, because...
Describe what is happening in the particle model below in the context of Boyle's law. (Assume that the picture on the left shows "before" and the picture on the right shows "after".)

As volume decreases, pressure increases.
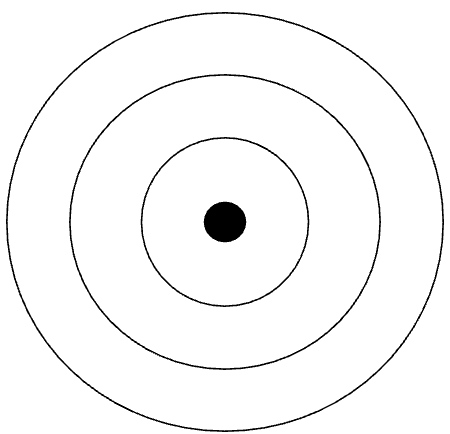
Draw an energy level model of an atom of nitrogen. (You can use the image below to help guide you.)

Element X has two natural isotopes. The isotope with a mass of 10.012 amu has a relative abundance of 19.91%. The isotope with a mass of 11.009 amu has relative abundance of 80.09%. Calculate the average atomic mass of this element.
10.8104 amu
What relationship do frequency and wavelength have?
indirect
__________________ is a property that describes the ability of a substance to be flattened into sheets without breaking.
Malleability
Why does an atom become positive when it loses electrons? Answer using a complete sentence.
An atom becomes positive when it loses electrons because there are more positive protons in the nucleus than negative electrons in energy levels. This makes the overall charge of the atom positive.
Would an ionic bond form between an atom of oxygen and chlorine? Why or why not? Explain!!
An ionic bond would not form between oxygen and chlorine, because they are both nonmetals.
Does crushed salt or rock salt dissolve faster in water?
In the experiment above, the water would be which type of variable?
Control
While deflating a balloon, the volume of the balloon increases. Is this an example of Boyle's law? Explain why or why not!
No, because in Boyle's law the number of gas particles remain constant.
What does atomic number tell you about an atom?
The # of protons in the atom.
Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% has a mass of 32.971 amu and 4.22% have a mass of 33.967 amu.
31.997 amu
An electron falls from a high energy level to its ground state. Which color of visible light is most likely emitted when this occurs and why?
Violet or purple light is likely emitted because it has the most energy.
____________________ is a property that describes the ability of a substance to be pulled into a thin wire.
Ductility
What is the difference between isotopes and ions?
Isotopes are elements with the same number of protons and a different number of neutrons. Ions are elements with the same number of protons and a different number of electrons.
Illustrate the ionic bond formation of sodium arsenide (Na3As) using Lewis electron dot diagrams.
Na1+[As]3-Na1+
Na1+