What charge does a neutron have?
neutral
What is the name of the element with 26 protons?
iron (Fe)
Identify the atomic number and the mass number in the isotope below.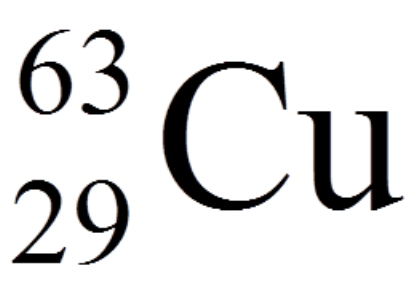
atomic # = 29
mass # = 63
What are the red arrows measuring?
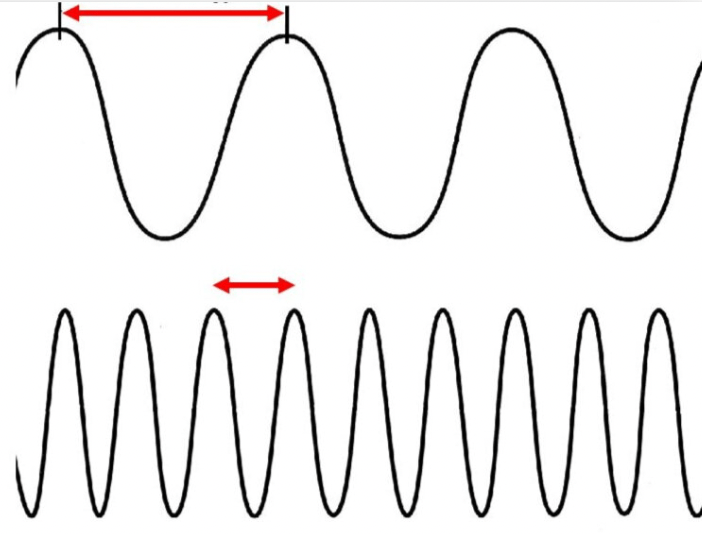
wavelength
How many electrons are in a neutral atom of barium?
56
Which subatomic particle has a charge of +1?
proton
What does atomic number tell you about an atom?
The # of protons in the atom.
Identify the atomic number and the mass number in the isotope below.
silver-107
atomic # = 47
mass # = 60
Which subatomic particle (proton, electron, neutron) is responsible for producing electromagnetic waves?
The movement of electrons is responsible for producing electromagnetic waves.
An isotope of calcium has a mass number of 41. How many neutrons does this isotope have?
21
What charge does an electron have?
-
How many electrons can the first energy level of an atom hold?
2 electrons; 8 for all other energy levels
What is the same about isotopes of the same element? What is different about isotopes of the same element?
The # of protons stays the same. The # of neutrons changes.
The energy of a wave of orange light and a wave of blue light are measured. Which wave has the lower energy and why?
Orange light has a lower energy because it has a longer wavelength.
What form of light are human eyes able to detect?
visible light
Where are electrons, neutrons, and protons located within the atom?
electrons = energy levels outside of the nucleus
protons & neutrons = nucleus
How many protons does an atom with an atomic weight of 118.710 amu have?
50 protons
Write the following in isotope notation:
protons = 32
neutrons = 40
electrons = 32
germanium-72
72/32 Ge
What is the difference between absorption and emission of energy?
absorption = energy is absorbed; electron moves up
emission = energy is released; electron moves down
How many siblings does Ms. Fig have?
2
In a neutral atom, the number of electrons is equal to ___________________.
Draw an energy level model of an atom of nitrogen. (You can use the image below to help guide you.)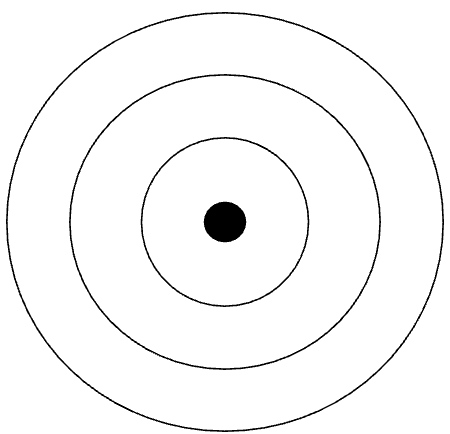

Element X has two natural isotopes. The isotope with a mass of 10.012 amu has a relative abundance of 19.91%. The isotope with a mass of 11.009 amu has relative abundance of 80.09%. Calculate the average atomic mass of this element.
10.8104 amu
An electron falls from a high energy level to its ground state. Which color of visible light is most likely emitted when this occurs and why?
Violet or purple light is likely emitted because it has the most energy.
What relationship do frequency and wavelength have?
indirect