The definition of the atom is this.
What is the smallest unit of matter? (variations can be accepted)
This type of variable is your input, something you change.
What is an independent variable?
This is the process by which two nuclei collide and join to form a larger nucleus.
Heterogeneous and homogeneous mixtures are distinguished based on this property.
What is our ability to separate the ingredients. (variations can be accepted)
This is the difference between elements and compounds.
What is, elements are composed of only one type of atom, compounds are composed of more than one?
This particle has no electric charge.
What is a / are neutron(s)?
You'd like to test how quickly you grow. You choose the days when you measure your height. Your height you measure is this variable.
What is the dependent variable?
This is the nuclear process which powers our power plants and atomic weapons.
What is nuclear fission?
Salt is dissolved in water. Salt water is an example of this.
What is a solution?
Hydrogen gas is made up of pairs of bonded hydrogen atoms (two hydrogen atoms bonded to each other). We thus call hydrogen gas this.
What is an element?
The nucleus comprises these two particles.
What are the neutron and proton?
On a graph, you plot your data for the independent and dependent variables. The independent variable is always plotted on this axis.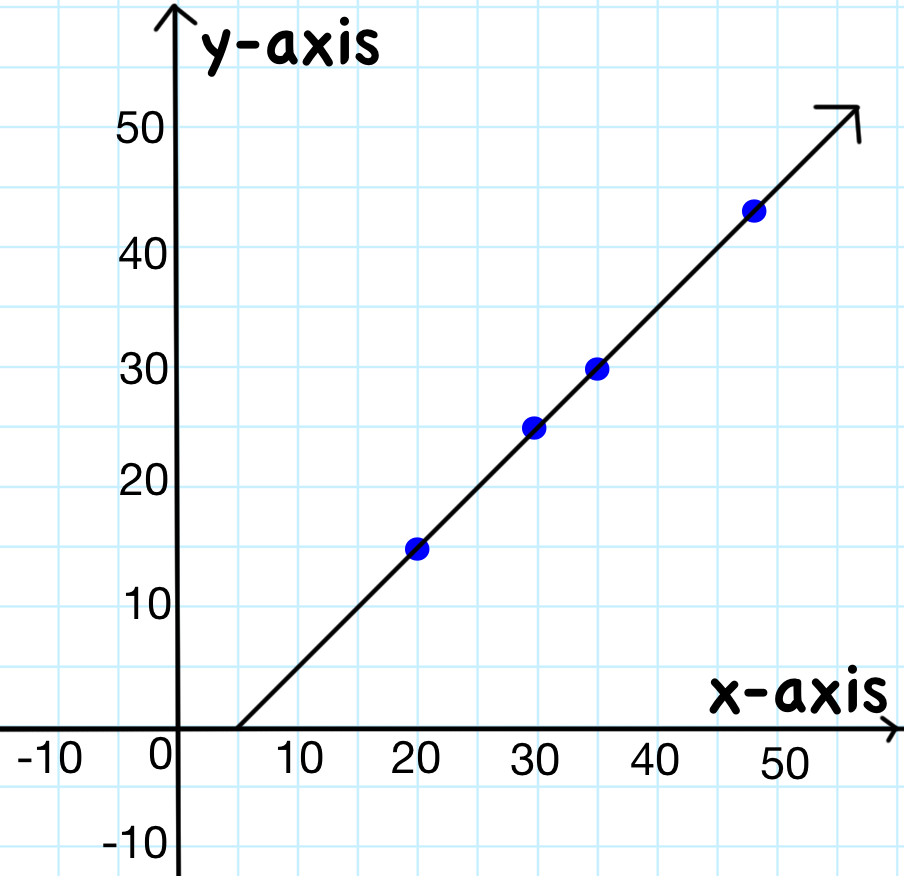
What is the x-axis?
An atom contains five electrons but only three protons. When protons and electrons aren't balanced, we call the atom this.
What is an / are ion(s)?
Salt is dissolved in water. The salt is called this component in the solution.
Water is made up of two hydrogen atoms and one oxygen atom bonded to each other. We thus call water this.
What is a compound?
These particles orbit the nucleus in these spaces.
What are shells/clouds/orbitals?
The (x, y) coordinates of the second blue point from the left.
What is (30, 25)?
What is the distance between the molecules/particles / the strength of their chemical/EM bonds / the way they fill a container?
This is the tool we use to separate sand from water.
What is filter paper?
We can categorize and look up elements in this famous chart.
What is the Periodic Table of the Elements?
The atom clearly has mass. Most of it is composed of this.
What is empty space?

What is 1?
The density of a substance is defined as the ratio of its mass to its volume. In other words, Mass/Volume. Respond to the three following statements: (1) mass is measured in these units; (2) volume is measured in these units; (3) as volume stays constant and mass increases, the density does this.
One difference between a solution and an homogeneous mixture is this.


What is/are the chemical bonds between the ingredients? (variations can be accepted)
What is the / are proton(s)?