a process in which one or more substances, the reactants, are converted to one or more different substances, the products
What are chemical reactions
Describe the three main states of matter.
What are solids, gases and liquids
an atom is made of …
What are Protons, neutrons, and electrons?
This speeds up a chemical reaction by lowering the activation energy, but it's not consumed by the process
What is a catalyst?
State the melting point of water.
What is 0 degrees Celsius?
Used to start a chemical reaction.
What is Catalysis
If the amount of this in a container is increased, the other increases. If the amount of this in a container is decreased, the other decreases
What is the relationship between the pressure-volume of a gas?
Things in which the electrons orbit around the nucleus
What are shells?
Compound produced after a chemical reaction
What is product
The law states that the property stays the same in an isolated system which evolves.
What is the law of Conservation of mass?
H2+ O --> H2O3
What is a non-balanced chemical reaction?
relationship between the pressure and volume of a gas
What is inversely proportional
Negligible
What is the weight of the neutron?
The relationship between the weights of reactants and the product before and after the chemical reaction.
What is stoichiometry?
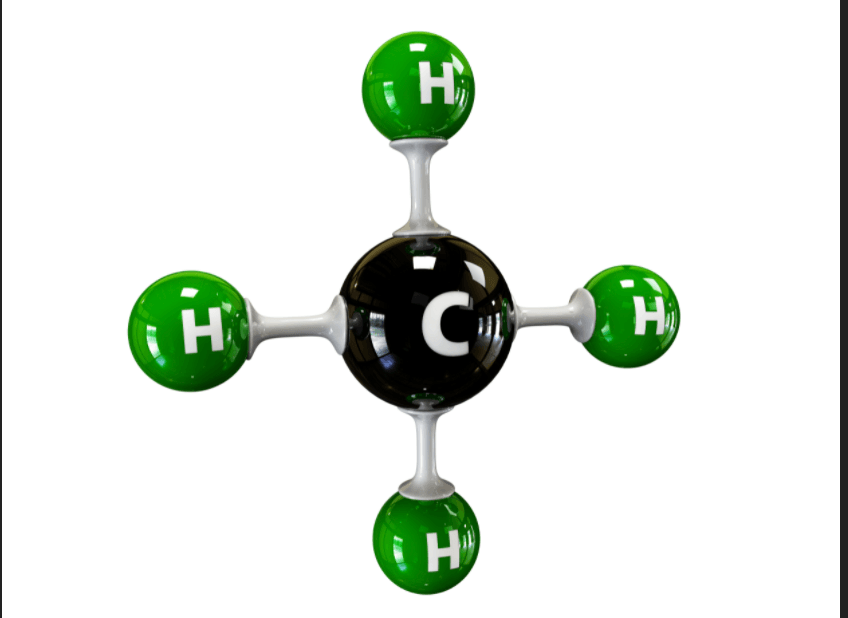 Methane, a greenhouse gas would be called by this chemical name and is produced in abundance by this animal.
Methane, a greenhouse gas would be called by this chemical name and is produced in abundance by this animal.
What is CH4 and cows? OR What is one carbon bonded to four hydrogen?
A state where the rates of the forward and reverse reactions are equal. It is represented with a double arrow in a chemical equation.
What is chemical equilibrium?
Thermal equation of state of ideal gases that states that the volume of a gas changes with temperature.
What are Charles' laws
In a chemical reaction, mass is either…
Created or destroyed
How heavy a mole of an element or a compound is.
What is the molar mass?
A gas will occupy all of this.
What is given space or volume?
When a substance changes its physical state from solid to liquid, liquid to gas, or vice versa, it is undergoing this type of change.
What is a physical change?
An egg will plop into a flask filled with steam when placed into a cold bath, due to a change in this variable.
What is a change in pressure inside of the container?
Increasing kinetic energy in a system is possible by decreasing this one thing.
What is volume?
A diver cannot breathe through a tube at depths greater than around 1 meter because of this.
What is increased water pressure?
 This is the reason a thermometer increases when placed into a warm or hot container.
This is the reason a thermometer increases when placed into a warm or hot container.
What is, The particles in the container transfer energy to the substance inside the thermometer, thus causing the particles in the thermometer to increase pressure and occupy more volume?
A covalent bond is a sharing of this.
What is a pair of electrons?
When a substance changes its physical state from solid to liquid, liquid to gas, or vice versa, it is undergoing this type of change.
What is a physical change?
This term refers to the process of a liquid turning into a solid.
What is freezing?
This property describes the ratio of a substance's mass to its volume.
What is density?
Different substances have different properties of matter which enable us to do what?
What is to tell them apart from one another?