Atom
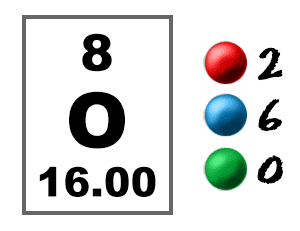
oxygen
Atomic number is determined by the number of these atomic particles
protons
Dissolving NaCl into H2O
Physical
Neutral
7 on the pH scale
these bonds are rude and tend to take electrons
ionic
Mg + P4 ---> Mg3P2
6Mg + P4 ---> 2Mg3P2
footwear to avoid
open toe shoes
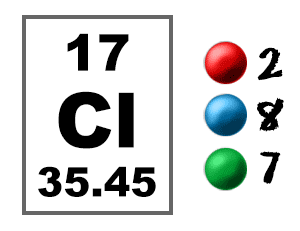
Chlorine
this atomic particle orbits the nucleus
electron
Boiling
Physical
Ranges from 0-14 and describes the amount of H+ ions
pH Scale

ionic
CH4 + O2 ---> CO2 + H2O
CH4 + 2O2 ---> CO2 + 2H2O
long hair should always do this
be tied back
hydrogen
contributes to the atomic mass with positive charge
proton
Decomposition of H2O2
Chemical
Has a high concentration of H+ ions
Acid
these bonds are polite and share well
covalent
N2 + H2 ---> NH3
N2 + 3H2 ---> 2NH3
how you should be takeing a whiff
wafting
atomic symbol "Na"
Sodium
neutrons
Combustion
Chemical
May be slippery
Base

covalent
NaCl + F2 ---> NaF + Cl2
2NaCl + F2 ---> 2NaF + Cl2
before mixing in chemicals, always do what?
check label again
contains 6 protons and 6 electrons
carbon
this particle may be shared or swapped to form compounds
electrons
Rusting
chemical
May taste sour
Acid
this bond is strong and requires a lot of energy to break
ionic
CO2 + H2O ---> C6H12O6 + O2
6CO2 + 6H2O ---> C6H12O6 + 6O2
three things you should wear in the lab
goggles, lab coat, and gloves
this noble gas has 10 protons and 10 electrons
Neon

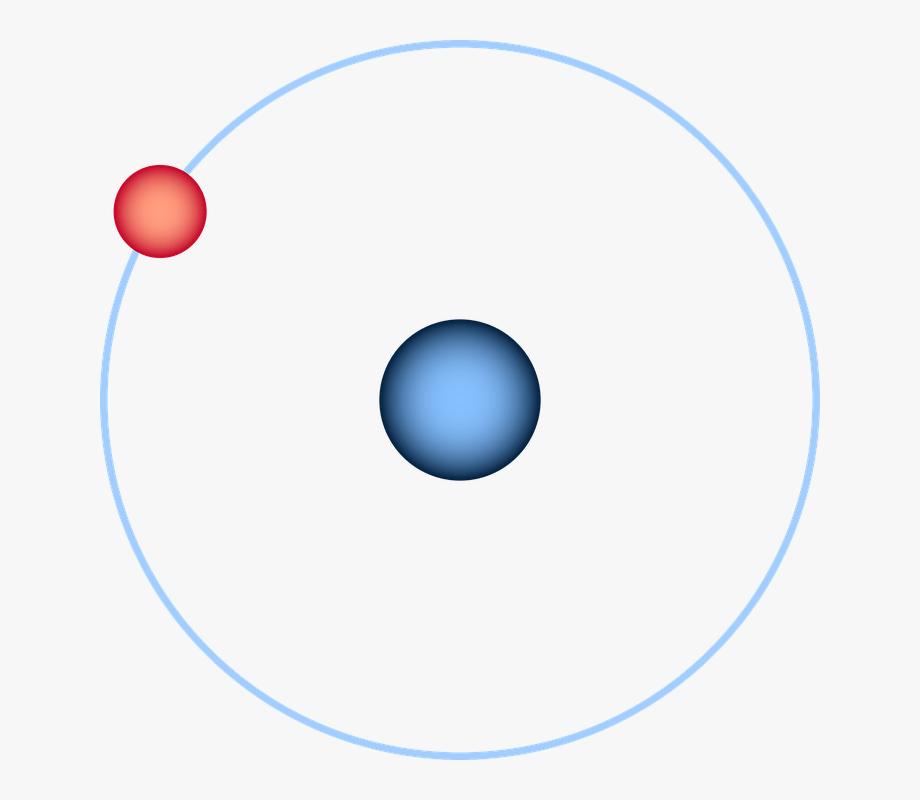
A - outer orbital
B - nucleus
C - electron
D - proton
E - neutron
F - orbital shells
Sublimation
Physical
Another word for "Basic"
Alkaline
this bond is weak and doesn't take much energy to break
covalent
P + O2 ---> P2O5
4P + 5O2 ---> 2P2O5
food or drink belong here
outside the lab