Which nuclear emission is listed with its notation?
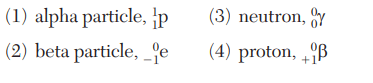
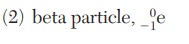
Complete the nuclear equation below for the decay of the Fe-59 used to diagnose anemia disorders, by writing a notation for the missing product.

cobalt-59
Identify the type of nuclear reaction represented by equation 1.

fission or transmutation
State the decay mode of tritium(H-3).
beta decay
Which statement describes a benefit of the nuclear reaction that occurs in a nuclear power plant?
(1) Large amounts of water are needed to cool the nuclear reactor.
(2) The power plant reaction can be used in dating geologic formations.
(3) Radioactive isotopes are stored for a very long time at the power plant site.
(4) A large amount of energy is produced from a small amount of a radioisotope.
(4) A large amount of energy is produced from a small amount of a radioisotope.
Identify the decay mode of Ne-19.
Positron decay
Which radioactive emissions are listed in order from greatest penetrating power to least penetrating power?
(1) alpha particle, beta particle, gamma radiation
(2) beta particle, gamma radiation, alpha particle
(3) gamma radiation, beta particle, alpha particle
(4) gamma radiation, alpha particle, beta particle
(3) gamma radiation, beta particle, alpha particle
Complete the nuclear equation for the alpha decay of Uus-294 by writing a notation for the missing product.

Uup-290
State, in terms of elements, why equation 2 represents a transmutation reaction.

An atom of iron is changed to an atom of cobalt.
Which radioisotope requires long-term storage to prevent the risk of biological exposure?
(1) N-16
(2) Pu-239
(3) K-42
(4) Au-198
(2) Pu-239
Which nuclide is paired with a specific use of that nuclide?
A) carbon-14, treatment of cancer
B) cobalt-60, dating of rock formations
C) iodine-131, treatment of thyroid disorders
D) uranium-238, dating of once-living organisms
C) iodine-131, treatment of thyroid disorders
Compare the amount of energy released by the fission of one mole of uranium-235 to the amount of energy released by the combustion of one mole of octane fuel, C8H18.
More energy is released during the fission reaction
Which nuclear emission has the greatest mass and ionizing power?
(1) alpha particle
(2) gamma radiation
(3) beta particle
(4) positron
(1) alpha particle
Which equation represents a nuclear fusion reaction?
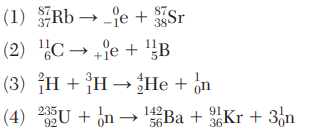
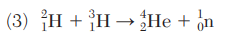
Identify the particle represented by X in equation 3.

neutron
Determine the time required for a sample of cesium-137 to decay until only 1/8 of the original sample remains unchanged.
90.6 years
Which risk is related to the radioactive isotopes used to generate electricity?
A) depletion of fossil fuels
B) depletion of atmospheric ozone
C) exposure to acid rain
D) exposure to nuclear emissions
D) exposure to nuclear emissions
State evidence that this nuclear reaction represents transmutation

— In this reaction, uranium is changing to other elements.
— Different elements are formed.
— One element becomes two new elements.
— Two atoms are formed with different atomic numbers from the U-235.
Which radiation has the least ionizing power and greatest penetrating power?
(1) alpha particles
(2) gamma emissions
(3) beta particles
(4) positron emissions
(2) gamma emissions
Complete the equation for the decay of phosphorus-30 by writing a notation for the missing product.

silicon-30
Identify the type of nuclear reaction that occurs when an alpha or a beta particle is spontaneously emitted by a radioactive isotope.
— natural transmutation
— transmutation
— nuclear decay
— radioactive decay
— decay
Determine the fraction of an original sample of the radioactive isotope I-131 used to test for thyroid problems that remains unchanged after 24.063 days.
1/8
Which radioisotope is used to treat thyroid disorders?
I-131
Write a notation for the missing product in equation 2.

xenon-142 or 142Xe
Compare the penetrating power of the alpha particles to the penetrating power of the gamma radiation.
The penetrating power of alpha particles is weaker than the penetrating power of the gamma radiation.
Complete the nuclear equation below, for the decay of cobalt-60 by writing a notation for the missing product.


Write a notation for the nuclide represented by missing product X in this equation.

— 144Ce
— cerium-144
— Ce-144
Determine the time required for an 8.00-mg sample of Sr-90 to decay until only 2.00 mg of the sample remains unchanged
58.2 y
Which radioisotope is used to determine the age of once-living organisms?
C-14
Ge-81 emits beta particles and has a half-life of 7.6 seconds. Determine the time required for a 16.00-gram sample of Ge-81 to decay until only 1.00 gram of the sample remains unchanged.
30.4s