Which type of nuclear radiation has the symbol
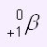
Positron

What is the difference between fission and fusion.
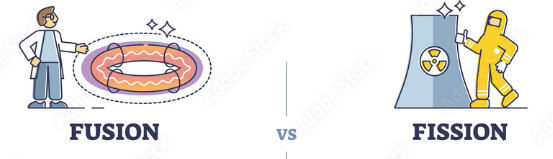
Fission "like division" is the process where an atom splits apart into two or more new atoms as products.
Fusion is the process where 2 or more nuclei fuse together.
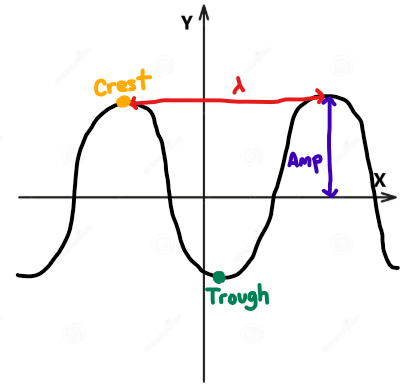
Define Amplitude by using 2 of the 3 vocabulary words in your definition:
Crest
Midpoint
Trough

Amplitude is the height of the wave from its mid-point to its crest or trough

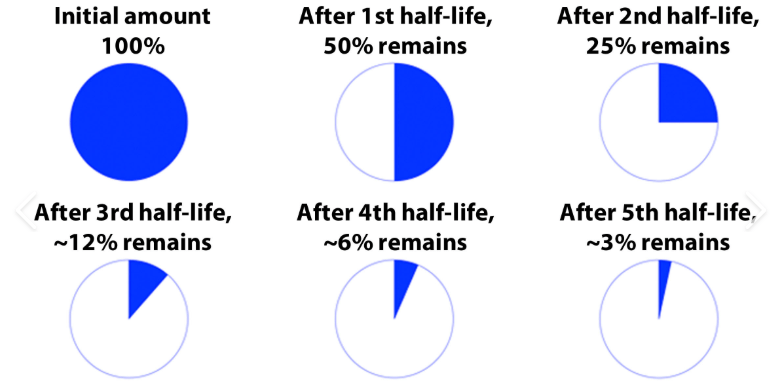
What is a half-life?


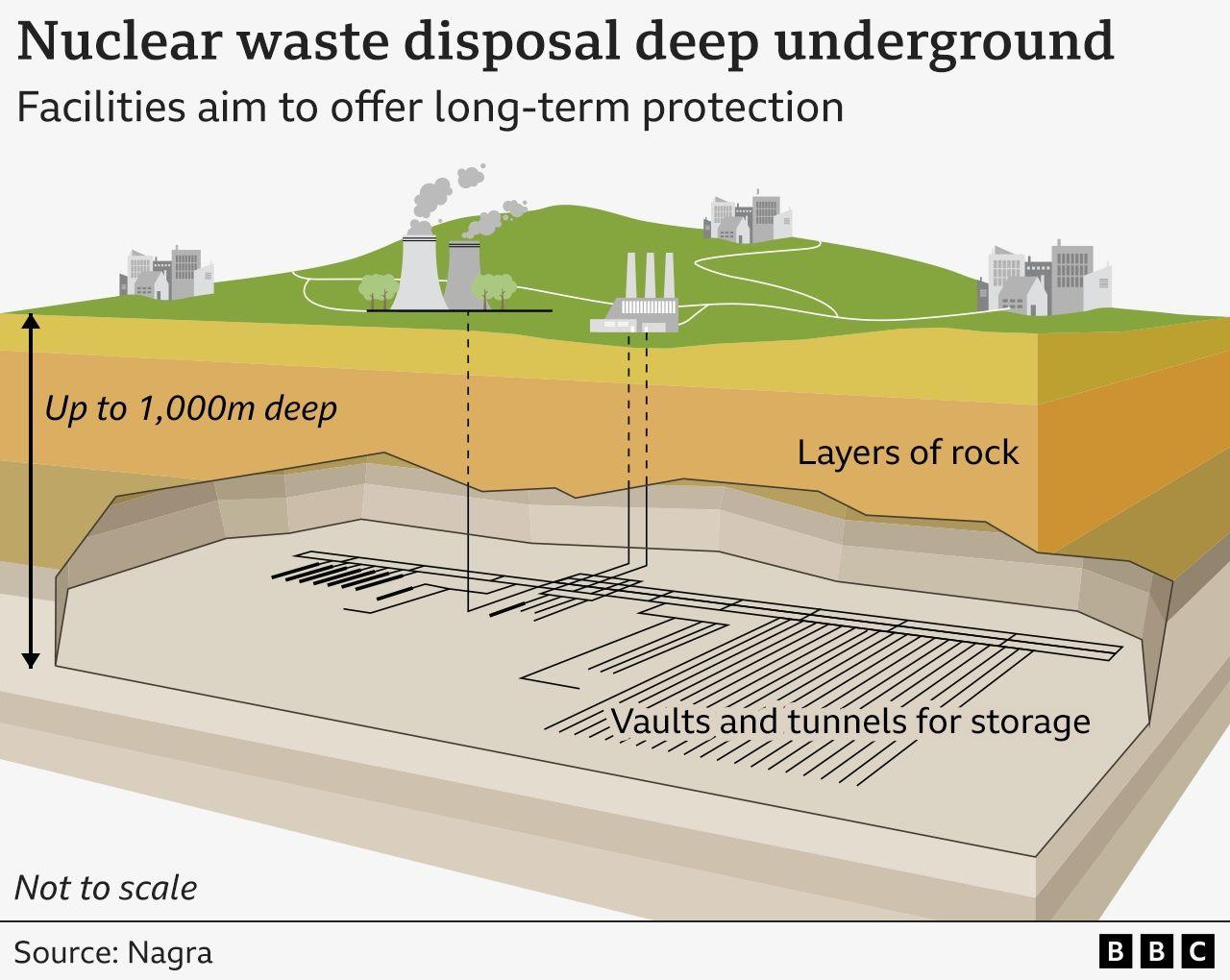
Why must nuclear waste be stored underground in a separate location after it has been used?

Nuclear waste is stored deep underground because it remains radioactive for long periods, and the thick layers of rock provide shielding that protects people and the environment from long-term ionizing radiation.
Which type of radiation has the nucleus of a Helium atom?

Alpha
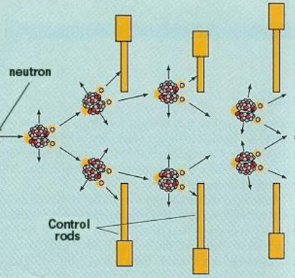
What do control rods do to neutrons in a nuclear reactor? How does this affect the function of the nuclear reactor?
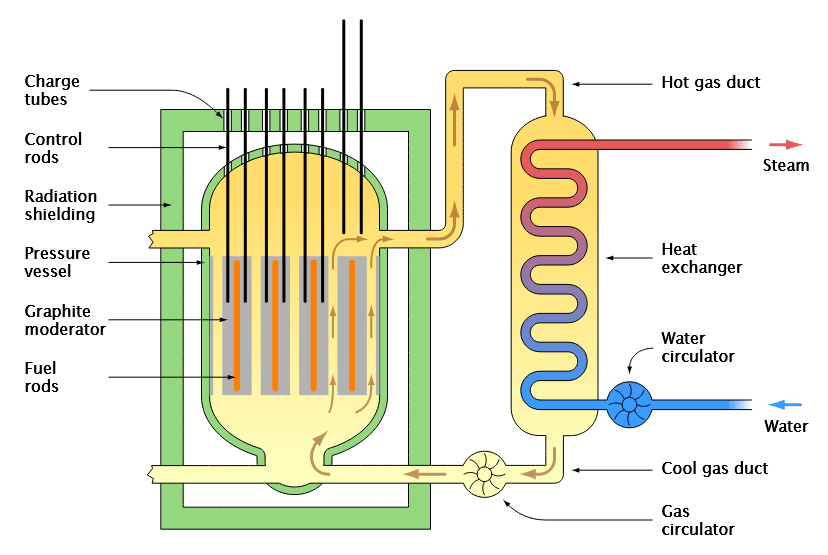
Control rods absorb neutrons, preventing them from combining with Uranium radioisotopes to undergo fission.
This process cools the nuclear reactor in return.
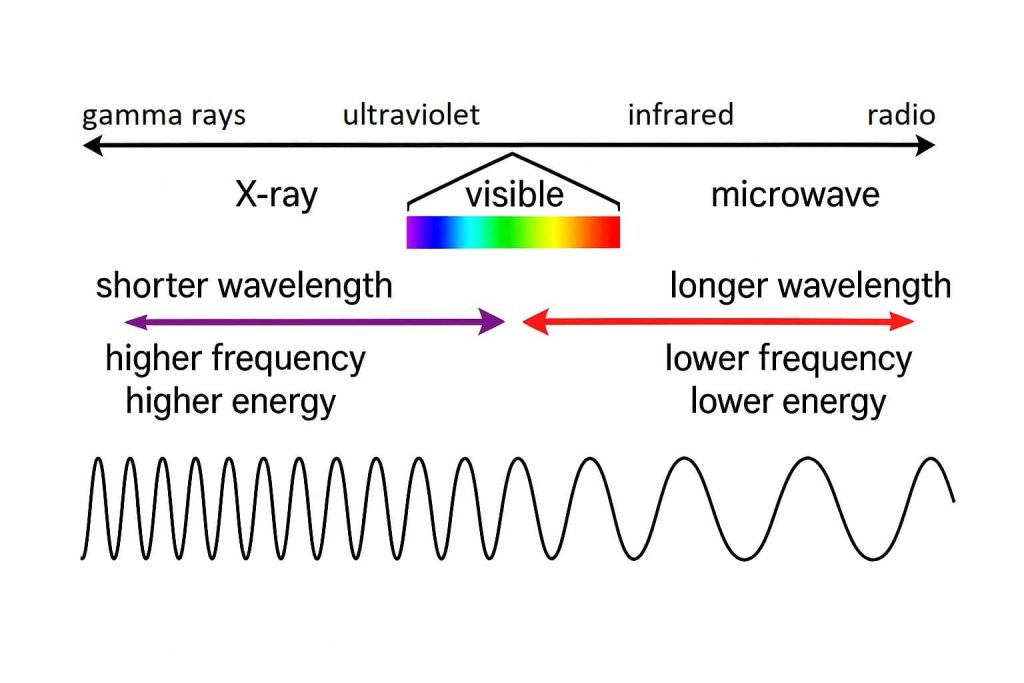
Which type of wave has the highest energy: Microwave, UV, or X-ray? Explain.
X-rays have the highest energy as they exist closest to the right side of the EMS where wavelength is short and frequency is high.
Short wavelength and high frequency means greater penetration power meaning greater energy.
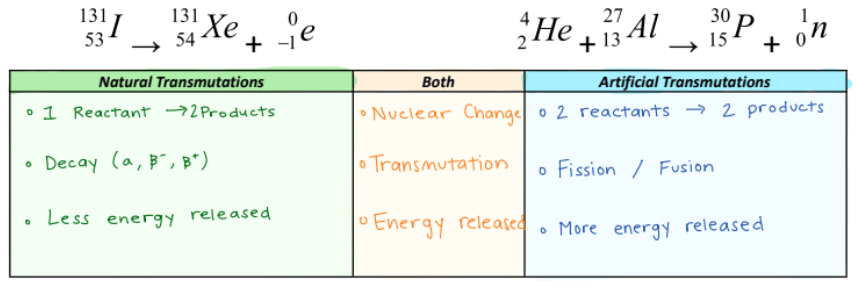
Identify two beta emitters from Table N and describe what changes during beta decay.
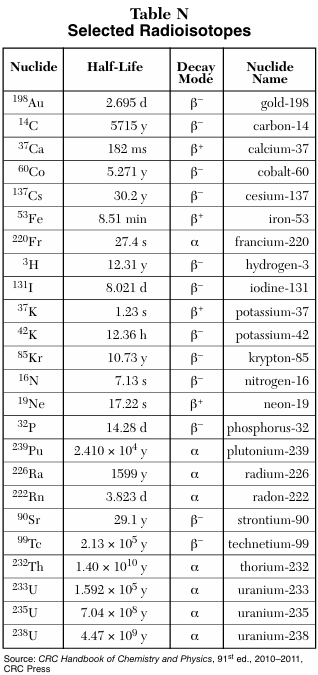
During beta decay, mass stays the same and charge decreases by 1.

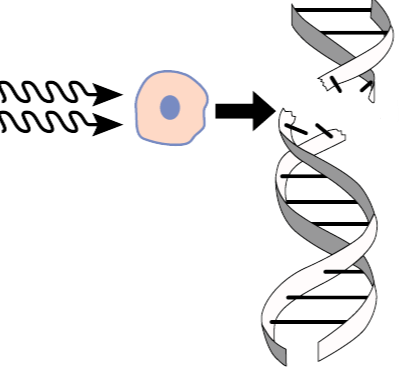
Why are gamma rays used to treat certain cancers, even though they can damage healthy cells?
Gamma rays are effective in treating cancer because their high-energy radiation breaks the DNA of tumor cells in ways that those cells cannot repair, while healthy cells are more capable of recovering.
Which nuclear particle bends away from the positive plate in a magnetic field?
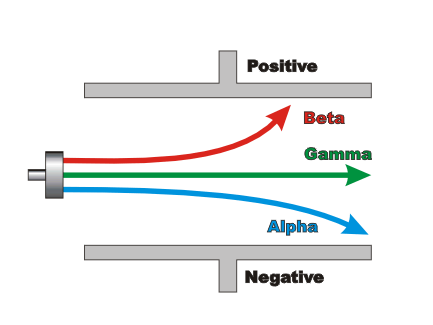
Alpha
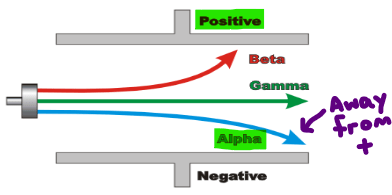
Explain how nuclear reactions obey the law of conservation of mass and charge.
Justify your answer by making your own nuclear reaction equation as an example.
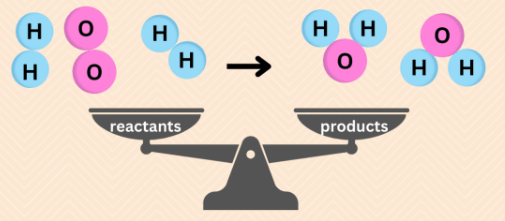
In nuclear reactions the mass and charge of the reactants and products are equal as the law of conservation states that mass and charge cannot be created or destroyed.
You finish with what you start with
Why can UV light cause sunburn, but visible light cannot?
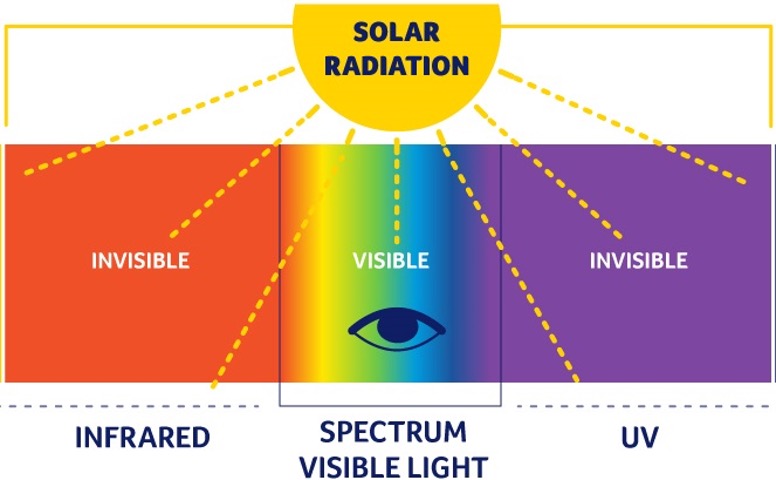
UV light has a higher frequency which means it has a higher energy. This energy difference allows UV light to damage our skin, compared to visible light which has a lower frequency.
Why are isotopes with short half-lives better for internal medical tracers.

Isotopes with shorter half-lives decay quickly, so the patient isn't exposed to radiation for too long.
It also minimizes the amount of radioactive material that remains inside the patient.
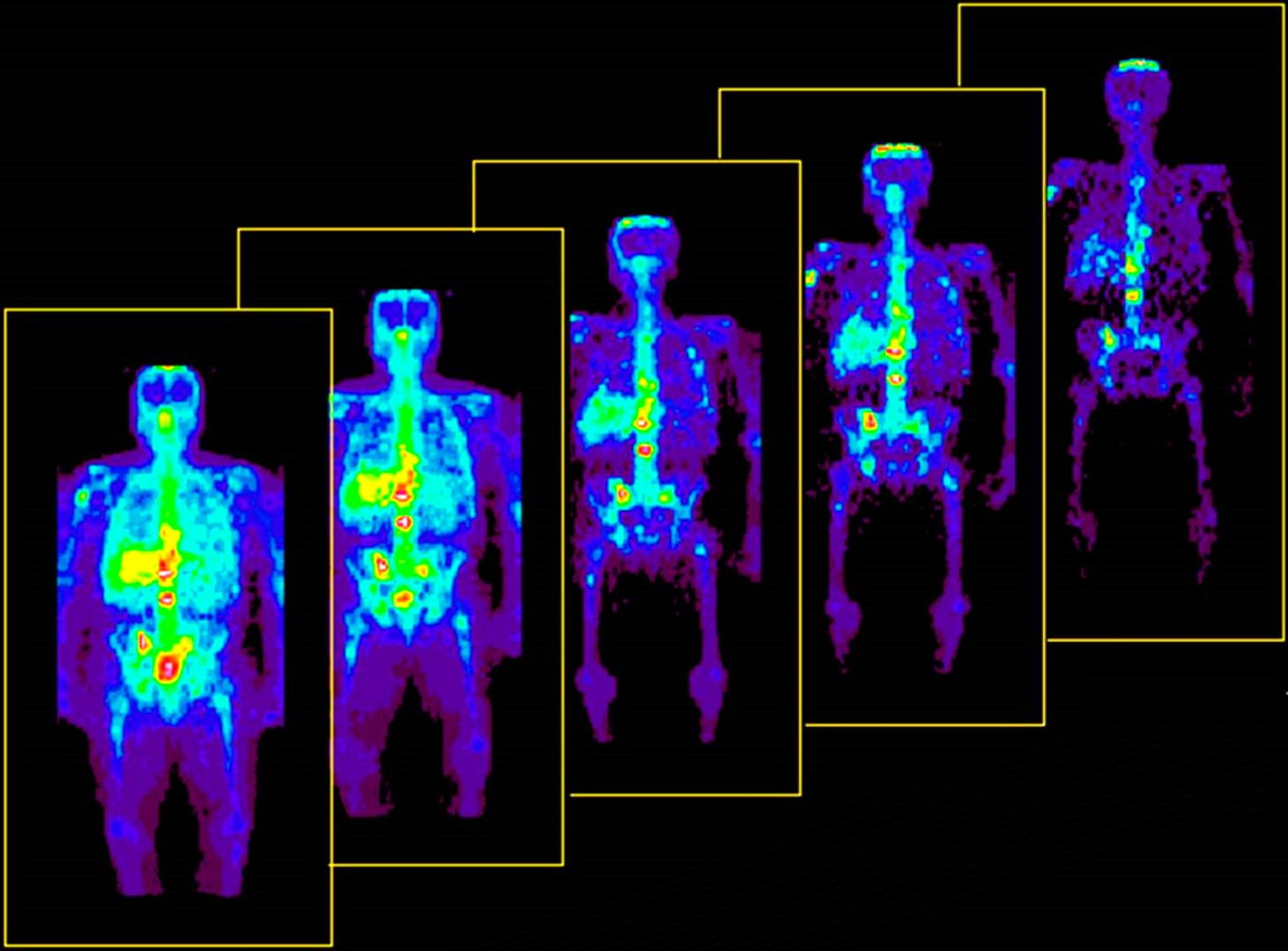
Why must X-ray technicians' step behind a lead wall when taking X-rays, while patients only receive a small dose?

Technicians step behind a lead wall to avoid repeated long-term exposure to ionizing X-rays, while patients receive only a single low dose needed for imaging.
X-rays cannot penetrate through lead, so they protect themselves behind a lead wall.

Explain why positron emission produces a new element, but gamma emission does not.
Positron changes the charge (# of protons) by -1
Gamma changes the charge (# of protons) by 0
Which reaction below is nuclear fission? Explain.
Equation 1:

Equation 2:
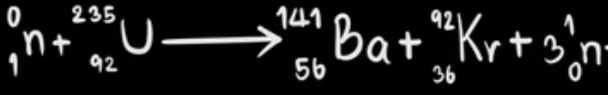
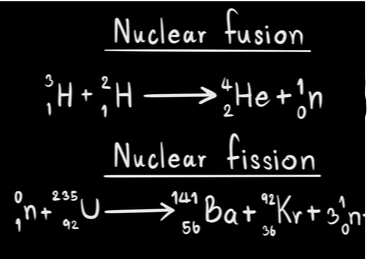
Equation 2

Fission "like division" is the process in which one atom splits into 2 or more new atoms as products.
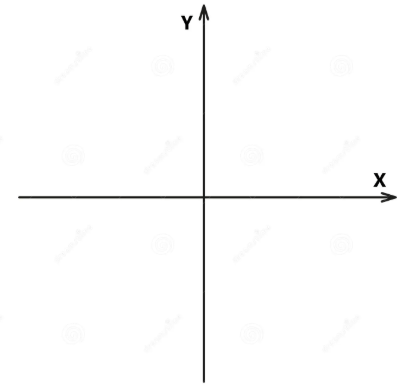
Develop a wave model of Radio Waves.
In your model be sure to include:
An X and Y axis
Wavelength
Amplitude
Crest
Trough
A fossil has 25% of its original C-14.
How many half-lives passed?
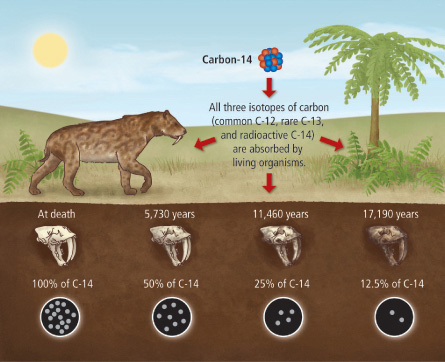
2 Half-Lives have passed.
Microwaves heat food quickly even though the container may stay cool. Explain how this is possible.
Consider how it relates to molecular interaction.

Microwaves cause water molecules in your food to rotate and vibrate, which produces heat.

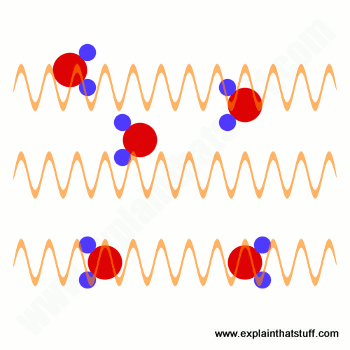
In terms of wavelength, frequency, and energy, why does gamma radiation require the most shielding?
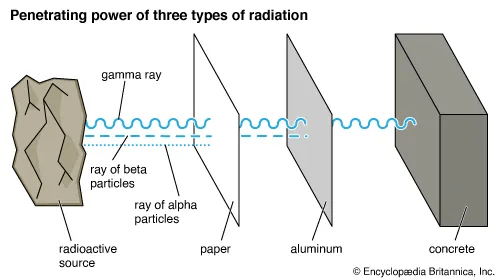
Gamma particles have short wavelengths and high frequency which mean they have more energy

Complete the following nuclear reaction:

Is this an example of natural or artificial transmutation?
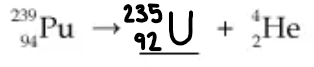
This is a natural transmutation. The Pu atom undergoes decay spontaneously with no other particle reacting with it.
When electrons jump to a higher shell, they absorb energy. When they return back to their original shell, they release this energy back. Explain why larger electron fall produces electromagnetic radiation with a shorter wavelength.
Consider the relationship between energy and wavelength.

The larger the fall, the more energy.
Short wavelengths hold much more energy than long wavelength.
A 64 g sample of Sr-90 decays to 16 g in 116.4 years.
Is this consistent with its half-life? Provide reasoning.

No, it is not consistent with its half-life.
It should be 4 g after 116.4 years.
Sr-90 has a half-life of 29.1 years.
116.4 years / 29.1 years = 4 half-lives
Start = 64 g
Half-Life 1 = 32 g
Half-Life 2 = 16 g
Half-Life 3 = 8 g
Half-Life 4 = 4 g
Being that it only decayed to 16 grams, that would of meant that it only experienced 2 Half-Lives or 58.2 years.
Provide your own example of a radioisotope that is used in real life.
What is the purpose of its function?

Possible Answers but not limited to:
Iodine-131 : cancer treatment / cancerous thyroid cells
Technetium-99m : imaging to detect heart disease and cancer
Cobalt-60 : radiation therapy for cancer
Carbon-14 : Radiocarbon dating to determine age of organic materials
Americium-241 : Used in smoke detectors
Uranium-238 : Used in Nuclear reactors