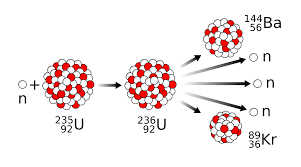
The splitting apart of breaking up of U-235 is defined as...
Nuclear Fission
How old is Kaos, the great dane?
4 years
In alpha Decay a __________ particle is released.
What is the last stable element on the periodic table of elements?
Lead (82 atomic number)
Any element above 82 tends to be unstable and thus radioactive.
222. Rn
86.
Nuclear Fusion
Radiation definition....
The penetrating rays and particles emitted by radioactive sources.
The alpha decay of Fr-211 results in:
He + At-207
Define Half-Life...
What is the time required for one-half of the nuclei of a radioisotope sample to decay into a new element.
0. B
-1
What subatomic particle is primarily responsible for causing chain reactions involved in fission?
Neutrons!
Radioisotopes definition...
The beta decay of Carbon-14
e + N-14
The half life of Carbon-14 is...
5730 years
241. Am
95
Both Fission and Fusion reactions release massive amounts of energy in the form of heat and gamma radiation. However, one of these two is more energetic than the other. Which process, fission or fusion releases more energy?
Fusion! It's what powers the sun and why hydrogen bombs are MUCH more scary than uranium bombs.
Carbon-14 is a radioisotope. Why?
Type of radiation that can penetrate through paper, wood and is stopped by concrete and lead.
Gamma radiation
Carbon-14 emits beta radiation and decays with a half life of 5,730 years. Assume you start with 100g of Carbon-14.
How much carbon is left after 1 half life?
What is 50g.
The half-life of Zn-71 is 2.4 minutes. If one had 100.0 g at the beginning, how many grams would be left after 7.2 minutes has elapsed?
7.2 / 2.4 = 3 half-lives
(1/2)3 = 0.125 (the amount remaining after 3 half-lives)
100.0 g x 0.125 = 12.5 g remaining
In the sun, ______________ atoms fuse together to create ______________ atoms, which produces a bunch of energy.
Hydrogen
Helium
The three most common types of Nuclear Decay are...
Beta Decay
Gamma Decay
Name the type of decay in the equation below
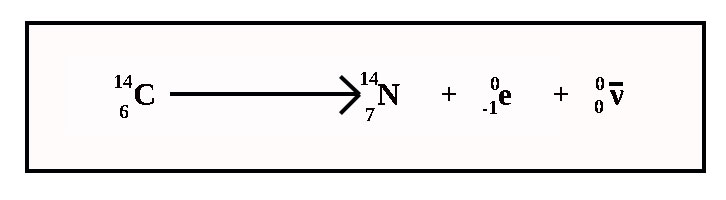
Beta + gamma
Carbon-14 emits beta radiation and decays with a half life of 5,730 years. Assume you start with 100g of Carbon-14.
How much carbon is left after 4 half lifes?
What is 6.25g
After 24.0 days, 2.00 milligrams of an original 128.0 milligram sample remain. What is the half-life of the sample?
The decimal fraction remaining:
2.00 mg / 128.0 mg = 0.015625
2) How many half-lives must have elaspsed to get to 0.015625 remaining?
(1/2)n = 0.015625
n log 0.5 = log 0.015625
n = log 0.5 / log 0.015625
n = 6
3) Determine the half-life:
24 days / 6 half-lives = 4.00 days