This German Physicist discovered X-Rays and noticed some materials darkened when electrons were shot at them.
Who is Wilhelm Roentgen?
or
Who is Roentgen?
This is the release of high energy particles (or rays) from a substance resulting in nuclei changes.
What is radioactivity?
These are the three common types of radiation.
What is Alpha, Beta, Omega.
This is the definition of a half life.
What is the amount of time needed for half of the original atoms to decay?
The amount left over from a 100g isotope with a 2 hour half-life, after FOUR hours.
What is 25 g?
This French Physicist found that rocks containing uranium salts emitted X-Rays NATURALLY, without needing electrons to be thrown at them!
Who is Henry Becquerel?
or
Who is Becquerel?
This is considered ONE wavelength.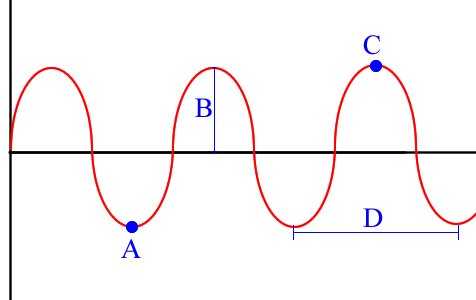
What is D?
This type of radiation has no mass and has the least amount of ionizing power.
What are Gamma Rays?
This is the isotope used to date the remains of old organic material.
What is carbon-14?
This isotope is the most radioactive out of this bunch:
a) half-life: 3400 years
b) half-life: 120 years
c) half-life: 8 years.
What is C?
or
What is half-life of 8 years?
This famous chemist isolated the minerals emitting x-rays, discovering that the URANIUM itself was the source of this phenomenon.
Who is Marie Curie?
This wavelength gives off the most amount of energy compared to the other wavelengths.
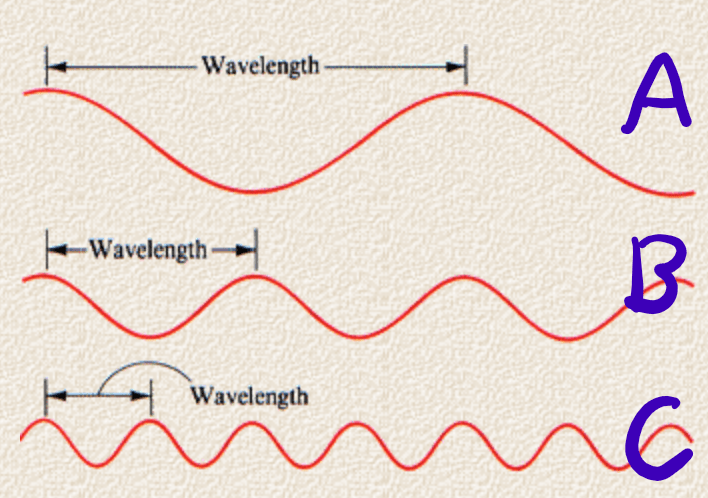
What is C?
This type of radiation can pass through skin and releases a fast moving electron from the nucleus.
What is Beta Radiation?
This is the limit for how old a sample can be before we need to use a different isotope to date it.
What is 50 000 years old?
Os-182 has a half life of 21.5 hours. After 3 half lives this much of a 10 gram sample will have decayed.
What is 8.74 g decayed?
Marie Curie coined this term for the process of atoms emitting x-rays.
What is radioactivity?
This type of atom has the same number of protons but a different number of neutrons.
What is an isotope?
Complete the following equation:
84Po --> 204Pb + ________
What is:
208/84 Po -> 204/82 Pb + 4/2 He
The name of this specific type of curve:
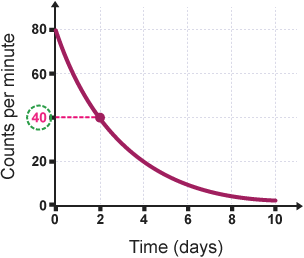
What is a Decay Curve?
You start with 100 g of an isotope. This isotope has a half life of 36 hours. When you only have 5 g left, this much time has passed.
What is 155.6 hours.
This University professor discovered that there were 3 common types of radiation. They also furthered our understanding of the Atomic Theory through the famous Gold Foil Experiment shown here...
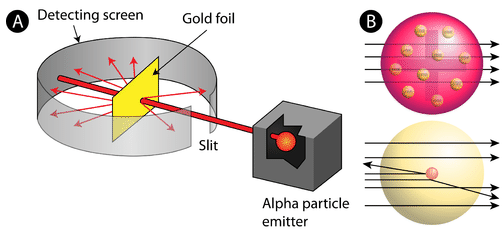
Who is Ernest Rutherford?
This is the purpose behind isotopes emitting radiation.
What is to become stable?
or
What is they are unstable?
The particle/ray that is given off in this equation:
20/9 Fe -> 20/10 Ne + ___
What is:
0/-1 e-
Pd-100 has a half-life of 3.6 days. If you start with 6.02 * 10^23 atoms,
How many atoms would be left after 18 days?
What is:
1.88*10^22 atoms.
The half life for a substance that after 24 days 2 mg remain from the original sample of 128 mg.
What is a 4 day half life?