Atomic Size INCREASES this way on the periodic table
DOWN a column OR Right to Left across a period
Most of the mass in an atom is located here
Nucleus
Substances that appear to the right of the arrow in a chemical equation
Products
___ is neither created nor destroyed during chemical and physical changes. It is only transferred.
Matter
The first element on the periodic table
Hydrogen
Elements in this group have full valence shells
Noble Gases
The negatively charged particles in an atom
Electrons
The small numbers that appear next to elements in a chemical equation that indicate the number of atoms in that molecule
Subscripts
This is considered the lowest point on the thermodynamic scale
Absolute zero
Out of Pentane and Octane, this compound has more carbon atoms
Octane
These elements have characteristics of metals and nonmetals
Metalloids
The positively charged particles in an atom
Protons
What is the molar mass of CaCO3
100g/mole
A molecule that can either donate a proton or accept an electron during chemical reactions
Acid
Organic compounds always contain this element
Carbon
What are the elements in Group 2 called?
Alkaline Earth Metals
The particles found in the nucleus of an atom
Protons and Neutrons
DRAW THE LEWIS STRUCTURE
Beryllium Chloride (BeCl2)
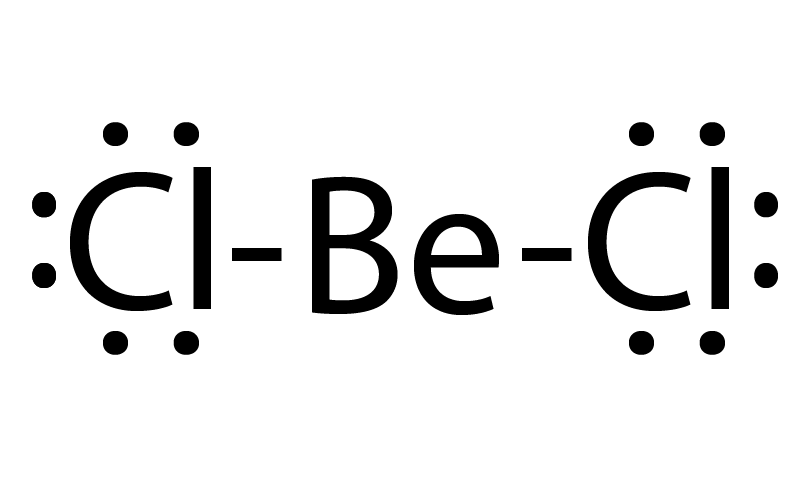
A simplified representation of the valence electrons in a molecule
Lewis structure
Substances that conduct electricity when dissolved in water
Electrolytes
The 4 elements are the building block elements in the human body
hydrogen, oxygen, carbon, & nitrogen
Iron reacting with nitric acid is this type of property
Chemical property
BALANCE THAT CHEMICAL EQUATION
___N2 + ___H2 ----> ___NH3
1 N2 + 3 H2 ---> 2 NH3
The temperature and pressure at which the solid, liquid, and gas phases are in equilibrium
Triple Point
Determine the chemical formula for Dinitrogen Pentoxide
N2O5