A 48-year-old woman with a prior history of a transient ischemic attack (TIA) presents to the emergency department with 1 hour of left-sided weakness and decreased sensation as well as a left-sided visual field defect. Her current daily medications include aspirin 81 mg and atorvastatin 40 mg. She is a nonsmoker. Her prior work-up included a negative hypercoagulable evaluation, echocardiogram, and a 30-day electrocardiogram monitoring.
The stroke team is activated. Her head and neck computed tomography angiogram (CTA) shows an acute right middle cerebral artery occlusion without evidence of other atherosclerotic disease. There is no hemorrhage. She is taken emergently for intracerebral thrombectomy with improvement in symptoms.
The echocardiogram performed at the time of the previous TIA is shown
Which of the following is the next best step in the management of this patient?
A. Agitated saline injection.
B. Carotid duplex ultrasound.
C. Inferior vena cava filter.
D. Oral anticoagulation.
E. Percutaneous closure.
The patient presented with an acute embolic stroke. The echocardiogram shows a shunt across the atrial septum most suggestive of a patent foramen ovale ([PFO] or possibly a small atrial septal defect). This can be a cause of an ischemic stroke due to paradoxical emboli from the venous system traversing the PFO and entering the cerebral arterial circulation. Given that this is her second event (prior history of a TIA and on aspirin) and she had an extensive negative work-up for other causes of stroke, this is a cryptogenic PFO mediated event; therefore she should be referred for percutaneous closure of the PFO.
The shunt is clearly seen on color flow Doppler imaging, therefore an agitated saline study is not necessary. Ruling out carotid disease is important in the evaluation of an ischemic stroke, however this patient had a neck CTA on presentation and therefore a carotid duplex ultrasound is redundant. An inferior vena cava filter can be used for treatment of lower extremity deep vein thrombosis when anticoagulation is contraindicated, however it is not indicated for the long-term prevention of recurrent strokes in patients with a PFO. There is no clear-cut benefit of anticoagulation over aspirin in patients with a cryptogenic stroke in the absence of atrial fibrillation.
![]()



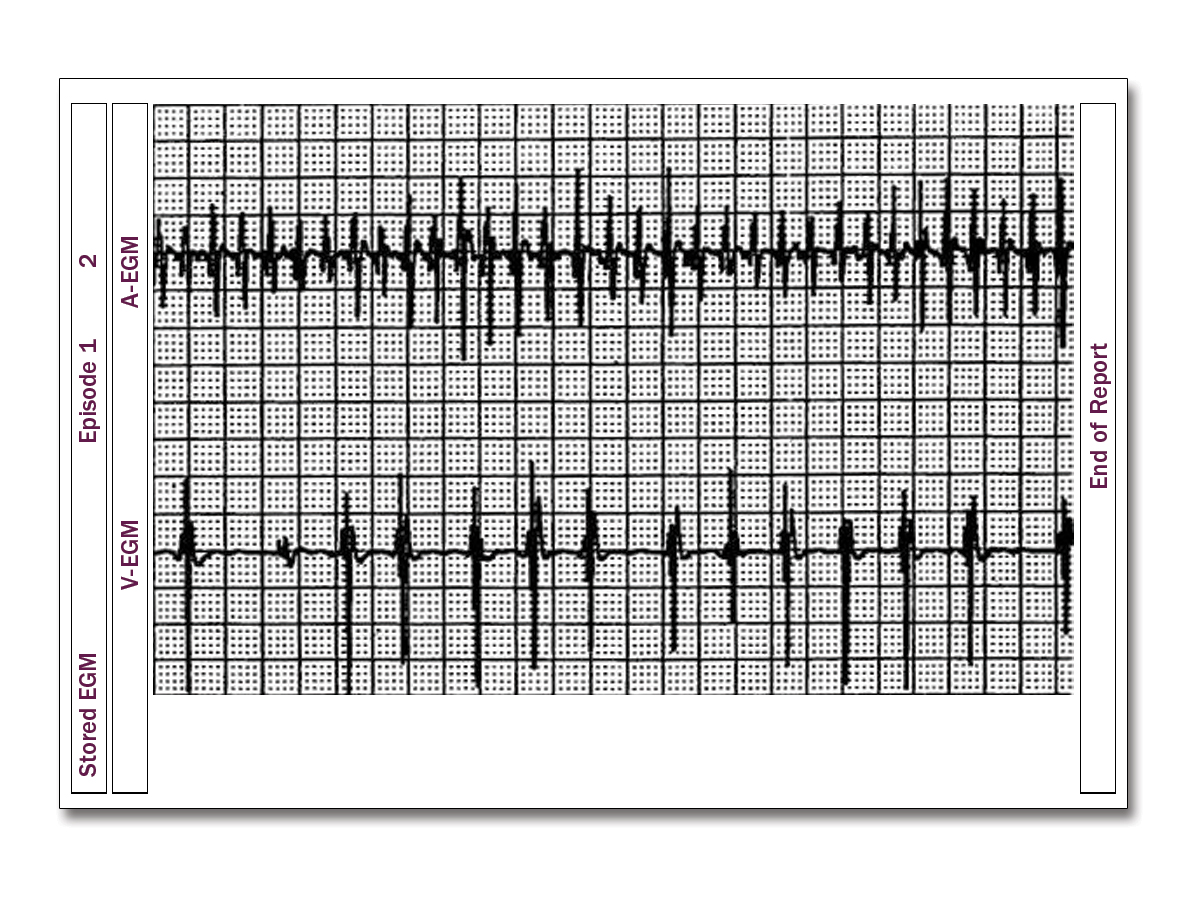
A 32-year-old man presents to the emergency department after his implantable cardioverter-defibrillator (ICD) fired three times while he was conscious. Prior to the event, he felt well with no complaints. His ICD was implanted 4 months earlier for a diagnosis of arrhythmogenic right ventricular cardiomyopathy (ARVC). He currently takes no medications.
He has a regular rhythm with heart rate of 75 bpm and blood pressure of 135/85 mm Hg. The remainder of the physical examination is unremarkable. Device interrogation for the period immediately preceding his shock is shown (Figure 1).
If an ECG was obtained immediately prior to the shock, which of the following rhythms would have been present?
A. Atrial fibrillation.
B. Ventricular tachycardia.
C. Ventricular fibrillation.
D. Sinus tachycardia.
E. Sinus rhythm.
This patient has ARVC. He had an ICD placed for primary prevention of sudden cardiac death. Device interrogation reveals more atrial beats than ventricular, indicating this was an inappropriate shock for supraventricular tachycardia (SVT), namely atrial fibrillation, which is common in patients with ARVC.
The irregularity of the ventricular electrograms in combination with the somewhat irregular and very fast rate of the atrial electrograms is consistent with atrial fibrillation. In rhythms such as sinus rhythm and sinus tachycardia, there would be a 1:1 relationship between the atrial and ventricular electrograms which is not seen in this tracing. Ventricular fibrillation would have very fast and irregular ventricular electrograms usually with a greater number of ventricular electrograms compared to atrial electrograms. Ventricular tachycardia would have fast regular ventricular electrograms and usually less and dissociated atrial electrograms.
A 63-year-old man is admitted to the hospital with a 2-week history of progressive shortness of breath, orthopnea, and edema. His past medical history includes diabetes mellitus, hypertension, and hyperlipidemia. His medications are amlodipine 5 mg daily, metformin 500 mg twice daily, and atorvastatin 40 mg daily.
On examination, his blood pressure is 112/88 mm Hg, heart rate is 102 bpm, and oxygen saturation is 94% on 2 L nasal cannula. There is jugular venous distension. Lung examination reveals bilateral crackles extending halfway up the posterior lung fields. Heart sounds are regular with a holosystolic murmur at the left lower sternal border. There is 2+ pitting edema to the knees.
His electrocardiogram shows sinus tachycardia with nonspecific T-wave changes. Laboratory results include sodium of 128 mEq/L, blood urea nitrogen of 62 mg/dL, and creatinine of 2.8 mg/dL (1.3 mg/dL 3 months prior). An echocardiogram displays a left ventricular ejection fraction of 35% and moderate tricuspid regurgitation.
Which one of the following is the most likely cause of this patient's kidney dysfunction?
A. Decreased renin secretion.
B. Increased intestinal perfusion.
C. Decreased vasopressin release.
D. Increased renal venous pressure.
E. Increased natriuretic peptide production
D
The correct answer choice is increased renal venous pressure.
Combined disorders of the heart and kidneys are classified as cardiorenal syndromes (CRS). Type 1 CRS refers to acute heart failure (HF) that results in acute kidney injury. It occurs in approximately one-fourth of patients admitted with acute decompensated HF, especially those with previous chronic kidney disease. Increased central venous pressure results in increased renal venous pressure and subsequent kidney congestion, which is an important hemodynamic determinant of CRS. Increased renin secretion occurs early in HF and stimulates sodium retention through angiotensin II and aldosterone. Additionally, the stress reaction to HF results in increased vasopressin release that stimulates water retention. These pathways, in turn, worsen systemic congestion and contribute to CRS. The natural compensatory and beneficial mechanism of increased natriuretic peptide production and resultant diuresis becomes overwhelmed in CRS. Finally, reduced perfusion of the intestine in HF due to shunting of blood results in release of endotoxins that have been proposed to worsen renal function.
A 75-year-old woman presents to the emergency department with severe substernal chest pain. Her past medical history includes hypertension, dyslipidemia, and prior carotid endarterectomy. Her medications are aspirin 81 mg daily, rosuvastatin 40 mg, carvedilol 6.25 mg BID, and hydrochlorothiazide 25 mg daily. Her vital signs are heart rate 98 bpm and blood pressure 169/102 mm Hg. Her physical examination reveals diaphoresis, a soft ejection systolic murmur, and palpable pedal pulses.
Electrocardiography reveals sinus rhythm with left ventricular hypertrophy but no acute ischemic changes. Troponins are normal. A chest X-ray is unremarkable.
A chest computed tomography (CT) demonstrates a crescentic, high attenuation area measuring approximately 3 mm in diameter in the posterior ascending aorta that does not enhance with contrast. There is no involvement of the great vessels or the aortic arch. There is no evidence of an intimal flap or compression of the lumen on this study. There is no pericardial effusion.
What is the best next step in this patient's management?
A. Emergent surgical consultation.
B. Coronary angiography.
C. Urine toxicology.
D. Intravenous hydralazine.
E.Transesophageal echocardiography.
The scenario describes a patient with ongoing symptoms due to an acute aortic syndrome. The CT report is suggestive of a type A intramural hematoma (IMH) and no evidence of intimal disruption. The definitive treatment for this pathology is emergent surgical repair due to the high risk of aortic rupture. The mortality of medical treatment alone may reach ≤40%. Hence, the most important next step is emergent cardiothoracic surgical consultation.
Intervention in the event of hemodynamic compromise is incorrect, as this may signify a life-threatening extension, including aortic rupture or dissection leading to tamponade.
Coronary angiography would delay definitive therapy and carries risks of worsening the acute aortic syndrome.
Urine toxicology prior to beta-blocker therapy may be considered if cocaine use is suspected in patients with hypertensive disorders and chest pain, but would not be the best choice with the imaging findings in this case.
The patient should undergo aggressive blood pressure control. Vasodilating drugs including hydralazine should not be given prior to adequate beta-blockers (Class III recommendation). Echocardiography is reasonable to evaluate for progression to aortic dissection with involvement of the aortic valve architecture. However, all these steps are adjuncts in management, with the most important step being emergent surgical repair.
A 20-year-old college sophomore presents to your office for evaluation of aortic root dilation. He had an echocardiogram after his internist heard a murmur, which revealed normal biventricular function with aortic root dilation of 5.0 cm (Z score 6.74). He denies chest pain, shortness of breath, palpitations, or syncope. He has a history of lens dislocation at age 17. He takes no medications. He is adopted and unaware of his family history.
What is the most likely diagnosis?
A. Marfan syndrome.
B. Loeys-Dietz syndrome.
C. Williams syndrome.
D. Noonan syndrome.
E. Ehlers-Danlos syndrome.
Marfan syndrome (MFS) is an autosomal dominant condition with mutations most commonly involving the gene (FBN1) encoding the connective tissue protein fibrillin-1. Classically, patients have ocular, cardiovascular, and musculoskeletal manifestations. Stringent criteria for the diagnosis of MFS (Ghent nosology) were proposed in 1996 and revised in 2010. The 2010 revised Ghent nosology puts greater weight on aortic root dilatation/dissection and ectopia lentis as the cardinal clinical features of MFS and on testing for mutations in FBN1 (Figure 1). This patient meets the criteria based on aortic root dilation (Z score ≥2; aortic root indexed to body surface area) along with lens dislocation. Alternatively, if there is no lens dislocation, a calculation of the systemic score would need to be calculated. A score over 7 with aortic dilation would also meet the criteria (www.marfan.org/dx/score).
The discriminating features of Shprintzen-Goldberg syndrome (mental retardation), Loeys-Dietz syndrome (bifid uvula, thin and velvety skin, easy bruising), and vascular Ehlers-Danlos syndrome (translucent skin, dystrophic scars, intestinal rupture) are not present. Loeys-Dietz syndrome is ruled out by the absence of other signs, including bifid uvula/cleft palate, arterial tortuosity, hypertelorism, diffuse aortic and arterial aneurysms, craniosynostosis, clubfoot, cervical spine instability, thin and velvety skin, and easy bruising. Williams syndrome is characterized by unusual facial features, intellectual disability, and hypercalcemia, not consistent with this patient's presentation. Noonan syndrome manifests as short stature and congenital heart disease, most often pulmonic stenosis.
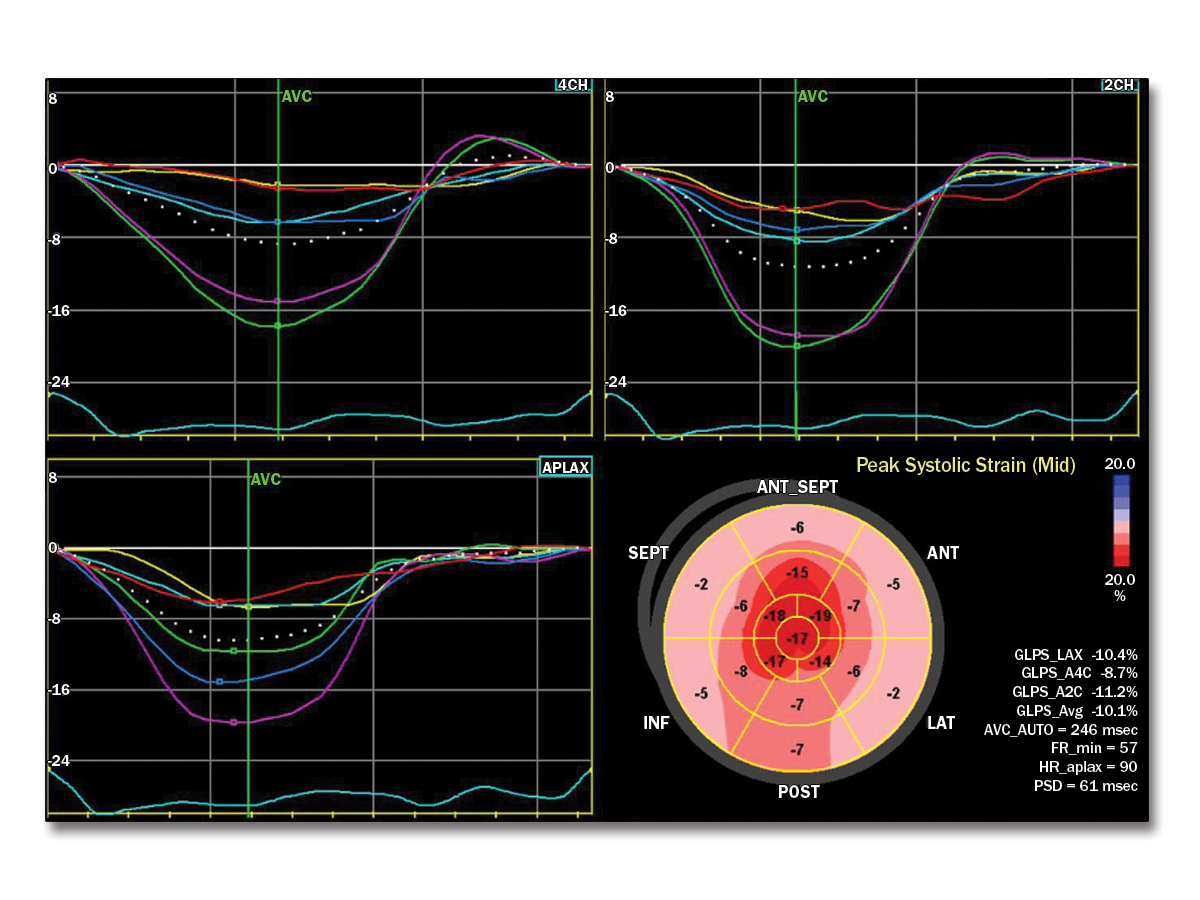
A 65-year-old man presents to the clinic with a 3-year history of progressive shortness of breath. He is initially diagnosed with exercise-induced asthma. His symptoms do not improve with treatment. An echocardiogram is performed that shows left ventricular hypertrophy and severe diastolic dysfunction with a right ventricular systolic pressure of 41 mm Hg.
Which one of the following is most likely to yield the correct diagnosis?
A. Technetium-99m pyrophosphate scan.
B. Hypertrophic cardiomyopathy gene panel.
C. High-resolution pulmonary computed tomography.
D. Coronary angiogram.
E. Exercise stress echocardiography
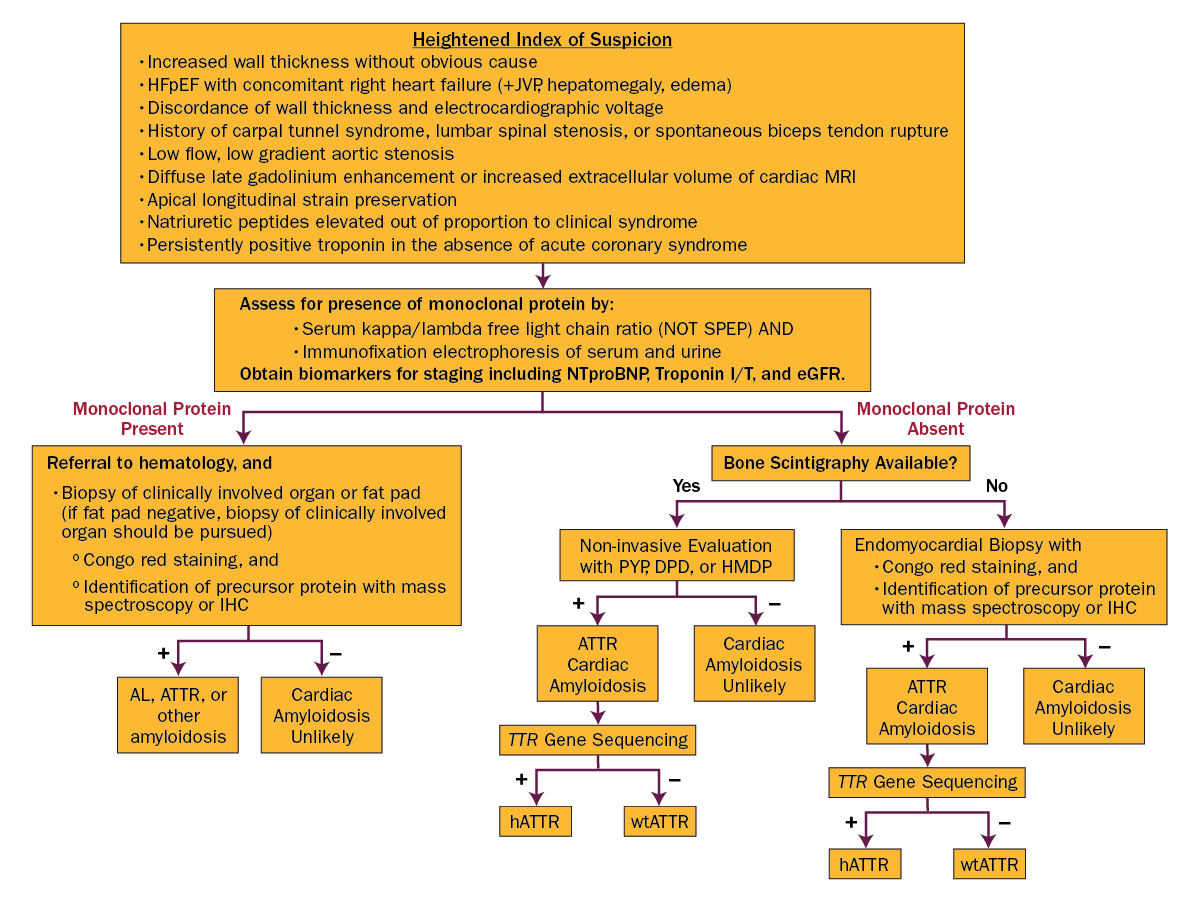
This patient has cardiac amyloidosis. Characteristic findings include biventricular increased wall thickness, biatrial enlargement without corresponding valvular lesions on echocardiogram, and low voltages on limb leads out of proportion to wall thickness. The ECG findings of a pseudoinfarct pattern of the anterior or inferior regions can also be seen in patients with cardiac amyloid. Key echocardiogram findings also include an apical sparing pattern with a 2:1 ratio on strain assessment. The strain pattern shown is the classic "cherry on top" pattern with severely diminished basal strain. Given this, the next step in diagnosis would be technetium-99m (Tc-99m) pyrophosphate (PYP) scintigraphy combined with serum and urine assessment of serum kappa/lambda free light chain ratio. Tc-99m PYP scans can be falsely positive in patients with light chain amyloidosis, and thus a diagnosis of transthyretin amyloidosis cannot be made without an assessment for plasma cell dyscrasias (Figure 3). In centers without PYP scanning, endomyocardial biopsy is recommended.
Hypertrophic cardiomyopathy is a less likely diagnosis given the patient's classic findings of the apical sparing pattern on the echocardiogram strain assessment and ECG demonstrating low voltage. Thus, the hypertrophic cardiomyopathy gene panel would not be indicated. Assessment of ischemia is likely to be of lower yield given these classic findings of amyloidosis, as outlined earlier. Given the echocardiogram and ECG findings, a pulmonary source for this patient's symptoms is less likely. Therefore, high-resolution computed tomography of the chest would be less helpful
A 45-year old woman was recently diagnosed with hypertrophic cardiomyopathy (HCM) after a syncopal episode. She is treated with a beta-blocker with improvement in her symptoms. You are considering an implantable cardioverter-defibrillator for primary prevention.
Which one of the following is associated with a higher risk of sudden cardiac death (SCD)?
A. Father with sudden death at 58 years of age.
B. Left ventricular hypertrophy with septal wall measuring 1.9 cm.
C. Presence of left ventricular outflow gradient
D. Supraventricular tachycardia on ambulatory monitoring.
E. Left ventricular apical aneurysm.
E
The correct answer choice is left ventricular (LV) apical aneurysm. The following factors increase risk for SCD:
- Family history of first-degree or close relative <50 years of age with SCD judged definitely or likely due to HCM
- Prior cardiac arrest or sustained ventricular arrhythmias
- Recent syncope suspected to be arrhythmic in origin
- Massive LV hypertrophy ≥30 mm anywhere in the LV wall
- LV apical aneurysm of any size
- End-stage HCM with LV ejection fraction <50%
- Nonsustained ventricular tachycardia on ambulatory monitoring
- Extensive late gadolinium enhancement on cardiac magnetic resonance imaging
A 50-year-old man presents to clinic for a routine pre-employment physical. He has no complaints. He has previously been told he has a benign murmur. He has a history of hypertension controlled with chlorthalidone 12.5 mg daily. He is a lifelong nonsmoker. His family history is significant for an uncle and two siblings with heart murmurs.
On physical examination, his heart rate is 70 bpm, blood pressure is 127/81 mm Hg, and oxygen saturation rate is 98% on room air. Cardiovascular examination reveals normal S1 and S2, as well as a grade 3/6 crescendo–decrescendo systolic ejection murmur at the right upper sternal border without radiation. The murmur increases in intensity with Valsalva maneuver and decreases in intensity with handgrip. There is an S4 gallop.
Which one of the following findings are expected on this patient's echocardiogram?
A. Congenital bicuspid aortic valve.
B. Calcified trileaflet aortic valve.
C. Dilated mitral valve annulus.
D. Asymmetric septal hypertrophy.
E. Subaortic membrane.
This patient has a physical examination that is most consistent with a dynamic outflow tract murmur that would support a diagnosis of obstructive hypertrophic cardiomyopathy (oHCM). The systolic ejection murmur of oHCM can be differentiated from the murmur of aortic stenosis or subaortic membrane based on physical examination maneuvers. The obstruction to the left ventricular outflow tract in oHCM is dynamic, as opposed to the fixed obstruction with valvular aortic stenosis and subaortic membrane. Subaortic membrane may also present with a murmur of aortic regurgitation. The degree of obstruction in oHCM, and therefore the intensity of the murmur, is dependent on preload. When preload is decreased (as with Valsalva maneuver), there is less volume flowing through the narrowed outflow tract, and therefore more obstruction, leading to an increase in murmur intensity.
Because this patient's murmur increased in intensity with Valsalva, he is more likely to have oHCM than aortic stenosis (either calcified or due to congenital bicuspid aortic valve). The crescendo–decrescendo nature and location of the murmur are not consistent with severe mitral regurgitation due to annular dilation, which gives a holosystolic murmur. An S4 gallop may be seen in all the answer choices except dilated mitral annulus.
A 72 year-old hypertensive man underwent a TEE due to severe back pain. The image below was obtained at the level of the upper descending thoracic aorta. Which of the following statements is correct regarding this image

A. There is an intramural hematoma with the arrow pointing to a penetrating ulcer
B. There is a complex atheromatous plaque
C. The thickening of the aorta is due to aortitis
D. There is an intramural hematoma with the arrow pointing to displaced intimal calcium
Choice D
The image shows an intramural hematoma with displaced intimal calcification. An aortic intramural hematoma is characterized by TEE as a circumferential or crescent shaped smooth margined thickening of the aortic wall without an intimal flap. The degree of aortic wall thickening is generally > 5mm. Intimal calcium may be displaced toward the lumen of the vessel by the accumulation of medial hematoma. Echolucent areas in the aortic wall may be seen suggestive of non-communication blood in the medial hematoma. An intramural hematoma is generally continuous over a relatively localized or extensive portion of the aorta and sometimes may coexist with regions of the aorta that have an intimal flap
A 23-year-old woman with Marfan syndrome (MFS) presents for preconception counseling.
On examination her height is 5' 8" and weight is 115 lbs. Her temperature is 98.4 degrees Fahrenheit, heart rate is 70 bpm, and blood pressure is 100/60 mm Hg. Her lungs are clear, heart sounds are regular with a mid-systolic click and II/VI systolic murmur, the abdomen is soft, and extremities are warm without edema.
Her echocardiogram showed an ejection fraction of 65%, mitral valve prolapse with moderate mitral regurgitation, and an aortic root diameter of 3.6 cm. On magnetic resonance imaging, the aortic root dimension was 3.7 cm and the rest of the aorta had a normal dimension.
With shared decision making, the patient elects to proceed with pregnancy.
During pregnancy, for which of the following complications is the patient at greatest risk?
A. Fetal complete heart block
B. Mitral valve chordal rupture.
C. Pulmonary embolism.
D. Aortic dissection.
E. Fetal Ebstein anomaly.
Aortic dissection is a rare, but often catastrophic event and occurs in patients with disorders of connective tissue, including those caused by fibrillin mutations such as MFS. Pregnancy is associated with a substantially increased risk of aortic dissection, probably caused by a maternal increase in blood volume, heart rate, and stroke volume, and by hormonally-mediated changes in the diseased aortic wall. The expected rate of aortic dissection may reach approximately 3% on average, ranging from 1% in women with an aortic diameter <40 mm to as much as 10% in high-risk patients (those with an aortic root diameter >40 mm, rapid dilation, or previous dissection of the ascending aorta). Despite the rare occurrence of aortic dissection in women with MFS and a normal-sized aorta, an event-free pregnancy cannot be guaranteed in these women. In patients with aortic dimensions >40 mm, it is reasonable to consider prophylactically replacing the aorta prior to pregnancy. In most women, aortic dissection occurs during the third trimester or postpartum, but it may occur at any time of gestation. According to the World Health Organization criteria, pregnancy is contraindicated in MFS patients with a dilated aorta >45 mm.
While patients with MFS may have mitral valve prolapse or chordal rupture, severe mitral regurgitation is not commonly observed in pregnancy. Ebstein anomaly can be a teratogenic effect of maternal lithium ingestion. The congenital heart block associated with neonatal lupus is considered a form of passively acquired autoimmune disease in which maternal autoantibodies to the intracellular ribonucleoproteins Ro (SS-A) and La (SS-B), cross the placenta and injure the previously normal fetal heart. While pulmonary embolism can occur during and after pregnancy, it is not associated with MFS.
A 42-year-old woman was admitted to the hospital with right-sided facial weakness and difficulty speaking. She has no past medical history. Computed tomography of the brain was negative for intracranial hemorrhage. Magnetic resonance imaging of the brain showed a large acute stroke in the territory of the left middle cerebral artery as well as a small old stroke in the territory of the right middle cerebral artery. She was successfully treated with endovascular thrombectomy. Her current vital signs are stable and her cardiac examination is unremarkable.
A transthoracic echocardiogram (TTE) showed normal left ventricular size and function, normal right ventricular size and function, and trace mitral and tricuspid regurgitation. The visualized portion of the aortic root was normal. No intracardiac masses were seen. A saline bubble study was negative at rest , with Valsalva, and with cough.
Which of the following is the most appropriate next step in the management of this patient?
A. Transcranial Doppler.
B. Cardiac computed tomography.
C. Right heart catheterization.
D.Transesophageal echocardiography.
E. Cardiac magnetic resonance imaging.
Transesophageal echocardiography (TEE) with agitated saline contrast, with provocative maneuvers such as coughing, Valsalva, or abdominal pressure (in sedated patients) is more sensitive for diagnosing intermittent interatrial shunting from a patent foramen ovale (PFO) and therefore the best answer. The finding of ischemic strokes of different ages in the territories of the carotid and middle cerebral arteries suggests cardioembolism as a cause of strokes, as opposed to lacunar strokes from small vessel disease or watershed territory infarcts from other causes. The sensitivity of TEE for diagnosing the cause of cardioembolic strokes is higher than TTE and is likely to be helpful if TTE is of poor quality in patient patients with stroke, in patients with stroke of unknown or unclear etiology, and in those with non-lacunar strokes. Additionally in this young patient, suspicion for paradoxical embolus via an atrial septal defect or PFO is high; therefore further investigation with a TTE is warranted. PFO is present in approximately 25% of the general population and up to 40% of younger patients with strokes.
Transcranial Doppler has high (>90%) sensitivity and specificity for right-to-left shunting, but is limited in many adults due to the absence of a suitable temporal bone window and also by the inability of transcranial Doppler to localize the anatomic origin of the shunt. Cardiac computed tomography (CT) can identify aortic atherosclerosis or intracardiac thrombus. The main limitation of CT is the lack of inherent soft-tissue contrast, which limits its assessment of the myocardium and identification of small thrombi. Intermittent shunting or opening of a PFO may not be detected using single-beat CT acquisition.
Cardiovascular magnetic resonance may have a role as an adjunct to echocardiography in selected patients with stroke, such as tissue characterization of cardiac tumors or masses seen with echocardiography or intracardiac thrombus evaluation, but does not currently have an evidence-based role in the routine evaluation of stroke.
Right heart catheterization can diagnose intracardiac shunts, particularly left-to-right shunt lesions, but is not sensitive for small intermittent shunting from a PFO. Additionally, right heart catheterization does not reveal structural details of the shunt lesion and is more invasive than the other options listed.
A 34-year-old woman is seen in your office complaining of palpitations. She has no prior medical history and takes no medications. She has no family history of heart disease. Her physical examination shows blood pressure of 98/78 mm Hg and heart rate of 62 bpm; it is otherwise unremarkable. An electrocardiogram (ECG) is obtained (Figure 1) and an echocardiogram is ordered
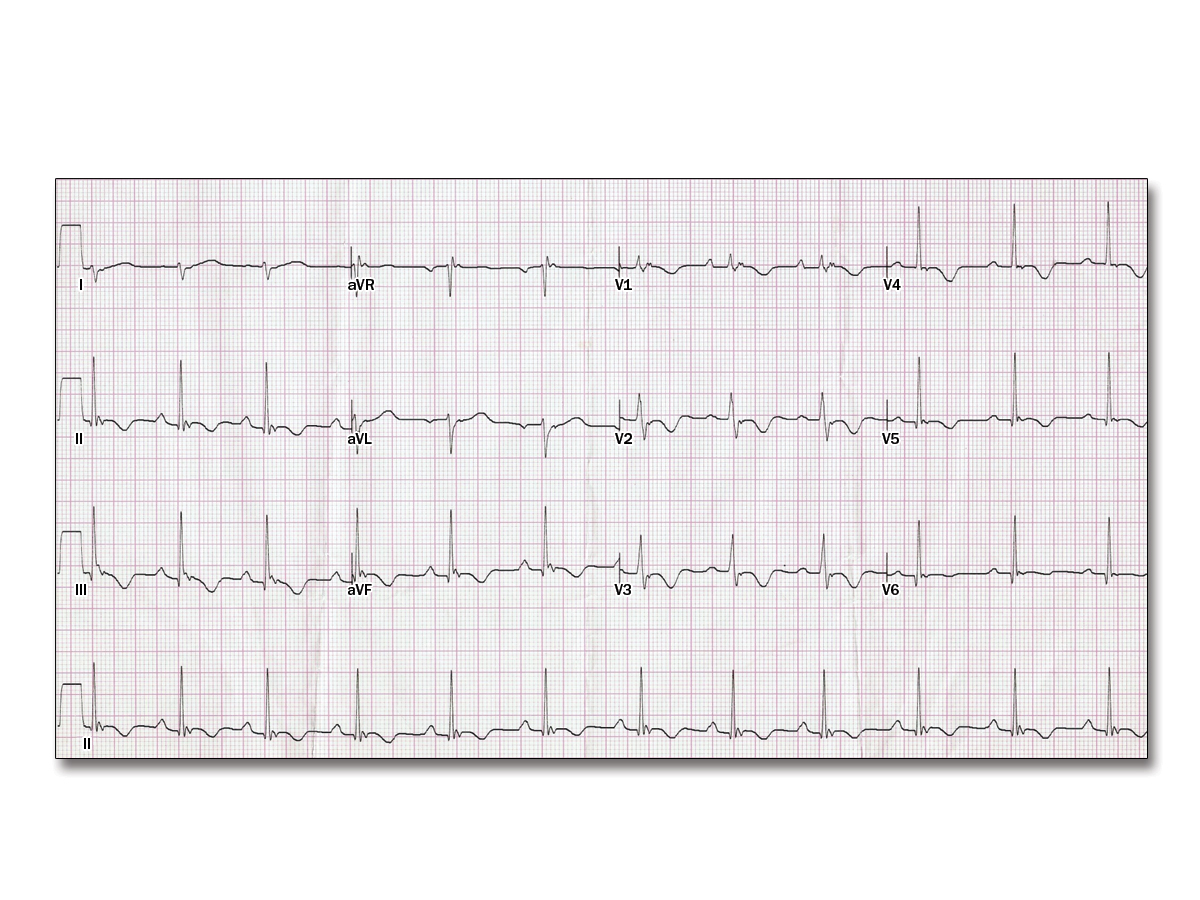
Which one of the following abnormalities will most likely be seen on this patient's echocardiogram?
A. Asymmetric septal hypertrophy.
B.Left ventricular apical ballooning.
C.Right ventricular dilatation.
D.Left ventricular noncompaction.
E.Secundum atrial septal defect.
C
The ECG (Figure 1) demonstrates classic findings of arrhythmogenic right ventricular cardiomyopathy (ARVC) with T-wave inversions in the anterior precordial leads and a high-frequency, low-amplitude deflection at the end of the QRS complex in many leads. These deflections are called epsilon waves. Physical examination is often normal in patients with ARVC and symptoms may not occur, but if present are most likely palpitations and syncope due to ventricular tachyarrhythmias. Echocardiography usually demonstrates right ventricular dilatation and right ventricular dysfunction.
Asymmetric septal hypertrophy would be seen in patients with hypertrophic cardiomyopathy; an ECG might show left ventricular hypertrophy. A secundum atrial septal defect may eventually cause right ventricular failure and is usually associated with a right bundle branch block or an incomplete right bundle branch block and right axis deviation, but is not associated with epsilon waves. Left ventricular apical ballooning is seen with takotsubo cardiomyopathy and is usually found in patients after a significant physical or emotional event; T-wave inversions are often seen across the precordium. Patients with left ventricular noncompaction usually have normal ECGs.
A 52-year-old man was admitted with acute decompensated heart failure (HF) to the cardiac intensive care unit 3 days earlier. Prior to admission, he experienced progressive dyspnea to the point that he was dyspneic at rest. He also experienced worsening lower extremity edema, orthopnea, and a 6.4 kg weight gain. His cardiac history is notable for a nonischemic cardiomyopathy diagnosed 4 months earlier. His left ventricle is moderately dilated with a left ventricular ejection fraction (LVEF) of 25%. He had no coronary disease on recent coronary angiography. Over the previous 3 months, he has been admitted to the hospital four times with decompensated HF despite compliance with dietary restrictions and guideline-directed HF treatment.
On examination, he is alert, oriented, and in no distress. His heart rate is 96 bpm with blood pressure of 88/62 mm Hg. Oxygen saturation is 95% on room air. Jugular venous pressure is elevated at 12 cm H2O. There are crackles at both lung bases. There are no murmurs and an S3 is noted. There is pitting lower extremity edema to just below the knees bilaterally.
He is currently on a dobutamine infusion at 5 mcg/kg/hour and a furosemide infusion at 20 mg/hour. He is net negative 4 L since admission. Laboratory studies reveal sodium of 124 mEq/L, potassium of 3.8 mEq/L, and creatinine of 0.9 mg/dL. An electrocardiogram (ECG) is obtained (Figure 1). Review of telemetry reveals 25 episodes of nonsustained ventricular tachycardia (NSVT), the longest lasting 28 beats at 162 bpm. A pulmonary artery catheter reveals the following: cardiac index of 1.7 L/min/m2, right atrial pressure of 8 mm Hg, pulmonary artery pressure of 34/16 mm Hg, and pulmonary capillary wedge pressure of 18 mm Hg.
Which one of the following is the most appropriate next step in the management of this patient?

A. Dopamine infusion.
B. Continuous venovenous hemofiltration.
C. Metoprolol succinate.
D. Coronary angiography.
E. Cardiac transplant evaluation.
The correct answer is evaluation for heart transplantation.
This patient with advanced HF with reduced ejection fraction improved clinically with intravenous inotropic support and aggressive diuresis. However, he has been admitted four times over a 3-month period despite adherence to dietary restrictions and guideline-directed medical therapy (GDMT). Based on his LVEF of <30%, New York Heart Association (NYHA) class IV symptoms, and frequent hospitalizations, he is considered to have American College of Cardiology/American Heart Association (ACC/AHA) stage D HF (Figure 2). According to the 2013 ACC/AHA Guideline for the Management of HF, evaluation for transplant is indicated in select patients with stage D HF despite GDMT, device therapy, and surgical management, where appropriate (Class I, Level of Evidence C). As he does not have coronary artery disease and does not meet criteria for cardiac resynchronization therapy given a narrow QRS on ECG, heart transplantation should be considered.
The patient is currently hemodynamically stable on dobutamine and is diuresing well, so the addition of dopamine is not indicated. Furthermore, dopamine may exacerbate ventricular tachycardia. Coronary angiography is unlikely to reveal new information that would alter clinical decision making at this time. As the patient has adequate urine output and stable renal function on intravenous furosemide, there is no indication for continuous venovenous hemofiltration at this point. Additionally, although this patient is having NSVT, the addition of a beta-blocker in a patient requiring hemodynamic support with an inotrope such as dobutamine, a beta agonist, is incorrect. Decreasing the dose of dobutamine and assessing hemodynamic response would be reasonable.
A 32-year-old man with a history of Marfan syndrome and thoracic aortic aneurysm presents after a recent chest computed tomography (CT) angiogram. He is 6'3" and weighs 193 lbs. His blood pressure is 130/78 mm Hg and heart rate is 55 bpm. Cardiac examination reveals pectus excavatum, normal S1 and S2, and no murmurs. Lungs are clear. Abdomen is thin with no organomegaly.
On CT, his aortic root measures 4.2 cm (compared with 3.9 cm the prior year), ascending aorta 4.5 cm, and aortic arch 3.3 cm.
What is the best next step in his care?
A. Add losartan 25 mg daily.
B. Add aspirin 81 mg daily.
C. Refer for surgical repair.
D. Chest computed tomography angiography in 3 months.
E. Transthoracic echocardiogram.
Marfan syndrome, affecting 1 in approximately 5,000 individuals, is an autosomal-dominant connective-tissue disorder due to mutations in the gene encoding fibrillin-1 (FBN1).
This patient would benefit from further blood pressure lowering to prevent progression of the aortic root dilation. This could be with either a beta-blocker or angiotensin II blocker. In a 2014 trial, patients with Marfan syndrome were randomly assigned to losartan or atenolol; they showed no differences in aortic dilation rate or presence of clinical events between treatment groups.
A repeat CT angiogram in 3 months is too soon and should not be performed again until 6 months.
Aspirin is beneficial for patients with aneurysms due to peripheral atherosclerosis to reduce cardiac events, but not in patients with Marfan.
An echocardiogram is not indicated since he had a recent chest CT for sizing of his aneurysm.
The threshold for surgical repair in patients with Marfan syndrome is an external diameter of 5 cm. Factors that will prompt repair at a diameter <5 cm include rapid growth of >0.5 cm in 1 year, family history of aortic dissection at a diameter <5 cm, or the presence of significant aortic regurgitation.
A 24-year-old male medical student has an echocardiogram during a practice session to acquaint students with the technology. The student has no prior medical history, takes no medications, and has no symptoms. The technician notices an enlarged right ventricle and measures it to be 36 mm at the base and 40 mm at the mid-cavity level. No atrial septal defect is noted on the transthoracic echocardiogram. The echocardiography attending confirms the findings and measures the right ventricular systolic pressure at 50 mm Hg. Agitated saline is administered and shows no interatrial shunting.
Which of the following is the next best step in the management of this student's care?
A. Repeat echocardiogram in one year.
B. An exercise stress test.
C. Right heart catheterization.
D. Cardiac magnetic resonance imaging.
E. V/Q scan.
Although a bubble study shows no evidence of shunting, this does not exclude all types of atrial level shunting or partial anomalous pulmonary venous return. Given his enlarged right ventricle, further investigation to exclude these is warranted. The best next step would be a cardiac magnetic resonance imaging (MRI) study and therefore a repeat echocardiogram in 1 year is not appropriate. Cardiac MRI would provide both physiologic and anatomic information including a Qp:Qs to guide further management. The definitive anatomy and diagnosis should be defined before proceeding with right heart catheterization. A V/Q scan would be indicated for the workup of pulmonary hypertension if a shunt lesion is not identified. An exercise stress test is not indicated in this asymptomatic individual for the evaluation of right heart enlargement.
A 66-year-old woman is seen in the office with shortness of breath that has been ongoing for about a year; the condition worsens when she is standing and improves with lying down. Her past medical history is notable for hypertension that is well controlled on amlodipine. On examination, she has marked kyphoscoliosis. Her resting oxygen saturation while breathing ambient air is 84% while seated and 93% when supine. A transthoracic echocardiogram reveals aneurysmal dilation of the ascending aorta with compression of the right atrium.
Which of the following is the next best step in the management of this patient?
A. An agitated saline (bubble) study.
B. Cardiac magnetic resonance imaging.
C. Right heart catheterization.
D. A cardiopulmonary exercise stress test.
E. Pulmonary embolism protocol chest computed tomography.
This patient has findings of platypnea-orthodeoxia syndrome (POS), characterized by dyspnea and hypoxemia in the upright position that resolve when lying supine. Potential etiologies of POS include intracardiac shunt caused by patent foramen ovale (PFO), atrial septal defect, or atrial septal aneurysm with fenestration as well as pulmonary conditions with ventilation-perfusion mismatch such as interstitial lung disease, hepatopulmonary syndrome, and pulmonary arteriovenous malformations. The most commonly described condition associated with POS is PFO. In order for a PFO to cause POS there must usually be an associated condition that causes distortion of the interatrial septum while in the upright position. Such conditions include post-pneumonectomy or lung transplantation, right hemidiaphragm paralysis, kyphoscoliosis, and ascending aortic dilation.
An agitated saline (bubble) study is the next best step to evaluate for a right-to-left shunt and should ideally be performed in both the supine and upright positions in a patient with suspected POS. While cardiac magnetic resonance imaging or transesophageal echocardiogram could provide additional anatomic information, an agitated saline echocardiogram is the most cost-effective, non-invasive diagnostic study.
Although chest computed tomography and right heart catheterization may demonstrate evidence of intracardiac shunting or help to quantify the degree of shunt, these are not the next best steps. A cardiopulmonary exercise stress test is not needed to make the diagnosis of POS or to establish the etiology. In patients with POS due to interatrial shunt without pulmonary hypertension, closure of this defect provides relief of symptoms.
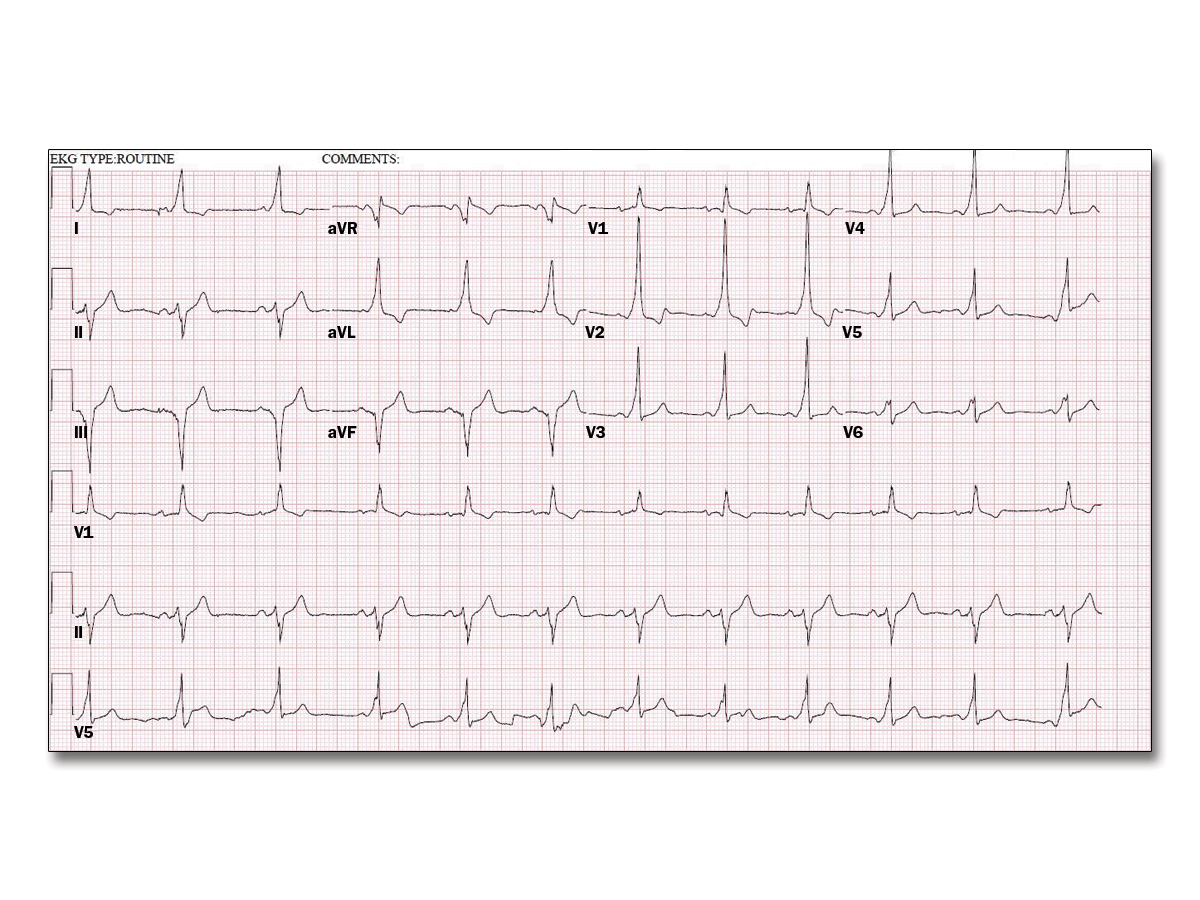
You supervised a treadmill stress test for a 36-year-old man with palpitations and a prior resting electrocardiogram (ECG) (Figure 1). He has no other medical problems and takes no medications; he has no known drug allergies. Six minutes into the Bruce protocol, he reported palpitations and another ECG was obtained (Figure 2). After stopping the treadmill and placing him on the stretcher, the rhythm continued. He was alert and said the palpitations were just like what he feels when he exercises at the gym. His pulse was 180 bpm and irregular, and his blood pressure was 104/60 mm Hg. Vagal maneuvers did not change the rhythm.
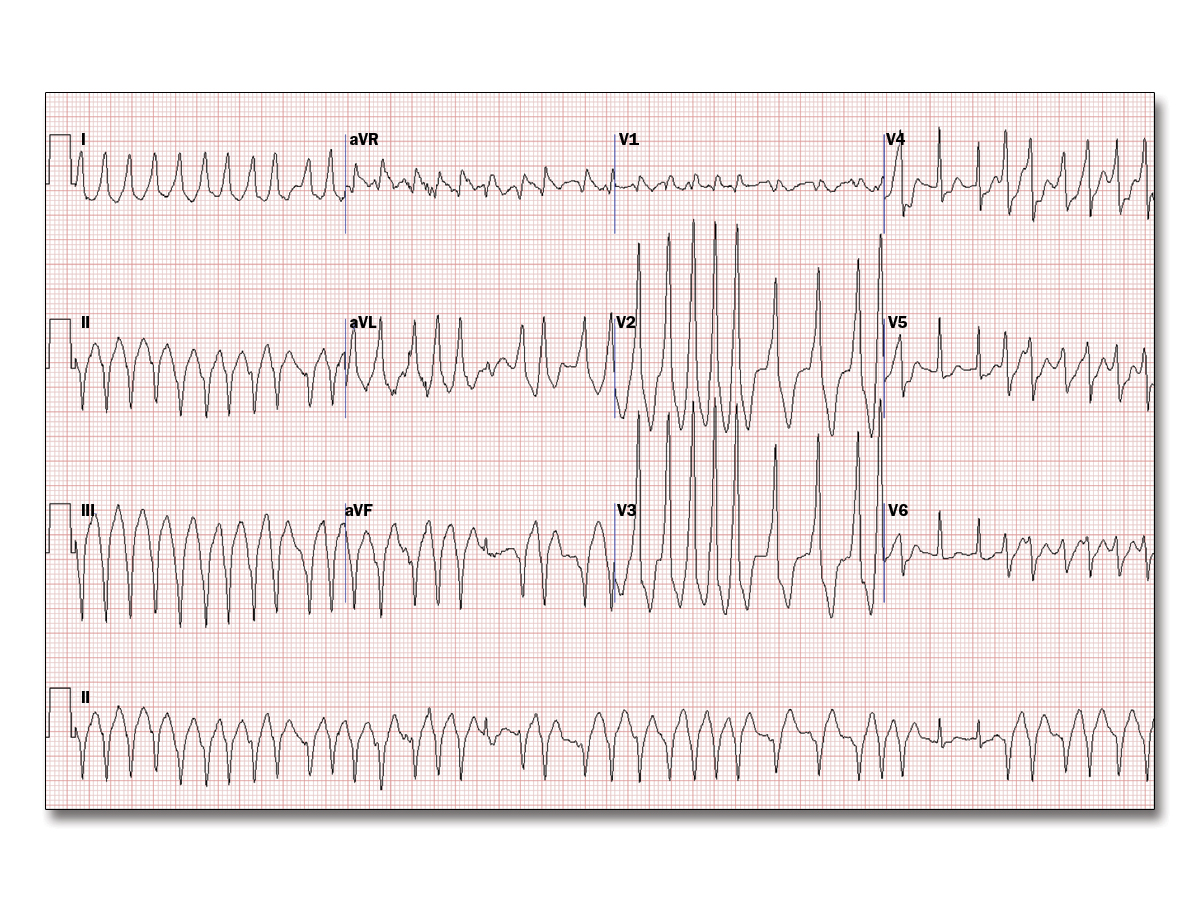
Which of the following is the best next step?
A. Metoprolol.
B. Lidocaine.
C. Amiodarone.
D. Magnesium.
E. Procainamide.
This patient with Wolff-Parkinson-White syndrome has pre-excited atrial fibrillation. Prompt treatment with intravenous procainamide, intravenous ibutilide, or synchronized cardioversion is critical to prevent deterioration to ventricular fibrillation; thus, procainamide is the best answer choice.
Agents that slow conduction through the atrioventricular node (such as beta-blockers, calcium channel blockers, or amiodarone) can lead to preferential conduction along the accessory pathway and may precipitate ventricular fibrillation.
Magnesium can be a useful adjunctive therapy for polymorphic ventricular tachycardia associated with long QT syndrome. There are case series of transient slowing of accessory pathway conduction after intravenous magnesium administration in sinus rhythm, but magnesium does not have an established role in managing pre-excited atrial fibrillation.
Lidocaine, a class Ic antiarrhythmic drug, is useful for substrate-mediated (monomorphic) ventricular tachycardia but does not treat atrial fibrillation. Additionally, lidocaine has been reported to accelerate accessory pathway conduction.
A 45 year-old male with recurrent diastolic heart failure and systemic symptoms including fever and night sweats underwent a TTE, based upon the image below which of the following about this patient's condition is correct?

A. RV involvement is common
B. MR is uncommon
C. LAP is usually normal
D. LV thrombus is uncommon
RV involvement is common. The patient has hypereosinophillic syndrome. This is a form of restrictive cardiomyopathy with endomyocardial involvement due to hypereosinophilia. There are 3 stages of disease due to toxic endomyocardial damage: (1) necrotic, (2) thrombotic and (3) fibrotic. If treated prior to the fibrotic stage, reversibility may occur. Echo findings include obliteration of the LV and RV apex size pressures with restrictive filling pattern. Diastolic function analysis is in contrast to constrictive pericarditis, with little change in respiratory variability and prominent diastolic reversal in the hepatic veins with inspiration. LV size and function are usually preserved. Additional findings include Merlon's sign which describes the hypercontractile basal segment in contrast to the hypokinetic apical segment.
A 37 year-old man underwent a routine outpatient TTE because of palpitations. His physical examination is unremarkable. An abnormality is noted which prompts a TEE. From the short and long axis views which of the following statements is correct about the abnormality shown?


A. It involves the left coronary cusp
B. It is usually a congential anomaly
C. Rupture would likely be fatal due to cardiac tapmonade
D. Rupture would likely be fatal due to aortic dissection
Choice B
The TEE images of the aortic root demonstrate a right coronary sinus of Valsalva aneurysm. Aortic sinus of valsalva aneurysms are most commonly of congenital origin although they can develop as a result of endocarditis, syphilis or trauma. Male predominance occurs with a male-to-female ratio of 3:1. The right sinus of valsalva followed by the non-coronary sinus is the most commonly involved sites. IF rupture occurs a communication develops from the right aortic sinus of Valsalva to the right ventricle or non-coronary sinus of Valsalva to the right atrium. Presentation includes a holosystolic murmur and shock but not tamponade
A 42-year-old woman presents with complaints of progressive shortness of breath. She recently immigrated to the United States and has not previously seen a cardiologist. She has three children, the youngest of which was born 8 years ago. She reports progressive fatigue and dyspnea over the past 6 months and over the last 2 weeks has noted the onset of peripheral edema. On review of systems she notes dizziness and near-syncope. On physical examination her vital signs are a blood pressure of 108/78 mm Hg, a heart rate of 95 bpm, and 92% oxygen saturation by finger oximetry. Cardiac auscultation is notable for a loud P2 component and a diastolic murmur heard best in the left upper sternal border. Cyanosis and clubbing are apparent in the toes but not the fingers. A chest X-ray shows cardiomegaly and prominence of the proximal pulmonary arteries. A 12-lead electrocardiogram shows normal sinus rhythm, biatrial enlargement, and biventricular hypertrophy.
Which of the following is the most likely cause of this patient's symptoms?
A. Secundum atrial septal defect.
B. Patent ductus arteriosus.
C. Ventricular septal defect.
D. Congenitally corrected transposition of the great arteries.
E.Tetralogy of Fallot.
The patient in this vignette has Eisenmenger syndrome as the result of an unrepaired patent ductus arteriosus (PDA). Clues to the presence of Eisenmenger syndrome in this vignette include features of pulmonary hypertension with a loud P2 sound on cardiac auscultation, radiographic prominence of the pulmonary arteries, and electrocardiographic evidence of right ventricular hypertrophy. Initially a PDA will produce a continuous "machinery" type murmur, however with the onset of shunt reversal this murmur may no longer be evident - as is the case in this patient. The finding of differential cyanosis (cyanosis and clubbing of the toes but not the fingers) is pathognomonic for PDA-level shunting. This is due to the fact that the right-to-left shunt at the ductus is distal to the subclavian arteries. Eisenmenger syndrome may result from any initial left-to-right shunt evolving over time to produce pulmonary vascular remodeling and pulmonary arterial hypertension, which then reverses the shunt to a right-to-left physiology. Examples include but are not limited to atrial septal defects, ventricular septal defects, and PDA.
Congenitally corrected transposition of the great arteries if it presents in adulthood would not result in shunting in the absence of other defects. Tetralogy of Fallot presenting in adulthood would result in cyanosis/Eisenmenger syndrome if not repaired, but not differential cyanosis.
32 year-old woman is referred for evaluation of rheumatic mitral valve stenosis. No Mitral regurgitation was noted. The following values were obtained by doppler echocardiography
E wave deceleration time 910 ms
Mean diastolic mitral gradient 17 mmHg
Diastolic mitral inflow VTI 66 cm
HR 85 beats/min
Which of the following statements is true?
A. MVA can be calculated by dividing 220 into deceleration time
B. SV across the mitral valve is 72 mL/beat
C. Pressure half-time is 355 ms
D. MVA is 0.8 cm2
E. During exertion, her mean gradient is expected to decrease
Answer D
A 43-year-old male with a history of tobacco use and hypertension presented to the emergency department with chest discomfort that radiated to the back. His chest pain began an hour prior to his arrival while eating dinner with his family. He denied any shortness of breath, palpitations, or nausea. His family history is notable for his father dying suddenly at age 50. On physical examination, he was diaphoretic. His heart rate was 90 bpm and blood pressure was 150/95 mm Hg. There were no murmurs and his lungs were clear. His abdomen was mildly distended with normal bowel sounds. His initial electrocardiogram showed normal sinus rhythm with no ST or T-wave abnormalities. His first troponin was negative. He was placed on a monitor. Ten minutes later, he became unresponsive. His rhythm strip was obtained (Figure 1).
Which of the following is the most likely etiology of the rhythm shown?
A. Long QT syndrome.
B. Brugada syndrome.
C. Acute myocarditis.
D. Myocardial ischemia.
E. Arrhythmogenic right ventricular cardiomyopathy.
Choice D
The rhythm strip shows ventricular fibrillation (VF). The most common cause of VF and polymorphic ventricular tachycardia is acute myocardial ischemia. The patient presented with chest pain due to an acute coronary syndrome. His ongoing chest pain with a normal ECG can be seen with an acute circumflex artery occlusion. He has multiple risk factors for coronary disease including tobacco use, hypertension, and family history of sudden cardiac death (presumably due to a myocardial infarction). Acute myocarditis, long QT syndrome, arrhythmogenic right ventricular cardiomyopathy (ARVC), and Brugada syndrome can cause VF but are less compatible with his clinical presentation.
A 38 year-old man presents to the emergency room following an episode of syncope preceded by palpitations. The patient denies chest pain or previous syncopal episodes. Telemetry monitoring reveals frequent PVC but no arrhythmias. ECG shows TWI in V2/V3 and a TTE is performed (see below).

Which of the following statements about this patient's condition is correct?
A. Associated LV dilated cardiomyopathy is common
B. Isolated RVOT dilation is a diagnostic feature
C. PHTN is common
D. IVC plethora is common
Answer B
Isolated RVOT dilatation is a diagnostic feature for ARVD which is a myocardial disease characterized by fibrofatty ventricular replacement, VT and SCD. TTE shows an aneursym of the apex in the RV, in the region of the "Triangle of Dysplasia".
Major criteria for ARVD include
1. RV akinesia, dyskinesis or aneurysm
2. PLAX or PSAX with RVOT dilatation
3. Reduced RV FAC (<33%).
Minor criteria for ARVD include
1. RV akinesis or dyskinesis
2. PLAX or PSAX RVOT dilatatin
3. RV FAC >33% but <40%
RV cardiomyopathy presenting as a dilated LV cardiomyopathy is well described but not common
A 47 year-old man presents with intermittent substernal chest pain during the preceding week. His past medical history is significant for arthritis for the past 15 years. On presentation he is afebrile and nontoxic appearing but his WBC is 20,000. Cardiovascular examination is notable for a grade II/VI early peaking systolic ejection murmur at the base and a grade I/VI decrescendo diastolic murmur radiating to the left lower sternal border. A TEE is performed to assess for endocarditis

Which of the following statements about the abnormality shown is correct?
A. Associated findings may include an aortic intramural hematoma
B. Associated findings may include a localized intimal dissection flap
C. Associated findings may include proximal aortitis
D. Associated findings may include mitral valve endocarditis
Answer C
The TEE image shows diffuse thickening of aortic-mitral curtain extending to the anterior leaflet of the mitral valve in a patient with aortitis. Although this finding may be associated with aortic valve endocarditis with periaortic root abscess, the clinical history is consistent with an inflammatory aortitis. The inflammatory process may involve the ascending aorta extending proximally to the sinuses of Valsalva, aortic leaflets, annulus and aortic-mitral curtain. Findings most commonly associated with aortitis include aortic aneurysms and aortic regurgitation. The TEE long-axis shows diffuse aortic wall thickening due to aortitis

You are seeing a 34-year-old man for follow-up of a ventricular septal defect. He has mild headaches most days and has leg cramps that are worse at night. He occasionally experiences blurry spots in his vision. He denies chest pain or shortness of breath. His current medications include amoxicillin 2,000 mg prior to dental work. His heart rate is 90 bpm, blood pressure is 102/62 mm Hg, and oxygen saturation is 93% on room air. His physical examination reveals a thin adult man. Mucous membranes are moist. His cardiac examination reveals a 3/6 holosystolic murmur. There is mild clubbing of the fingers. His hematocrit is 63%, hemoglobin is 21 g/dl, and platelet count is 101,000. His echocardiogram shows a large ventricular septal defect with bidirectional flow. Both ventricles are dilated with low-normal systolic function. The tricuspid regurgitant velocity is 4 m/s. The inferior vena cava is 2.1 cm in diameter, but does not collapse with inspiration.
Which of the following is the next best step in the care of this patient?
A. Phlebotomy.
B. Aspirin.
C. Uric acid.
D. Iron studies.
E. Hydroxyurea.
This patient has Eisenmenger's syndrome. This syndrome is associated with secondary erythrocytosis, thromboembolic events, cerebrovascular complications (stroke and brain abscesses), hyper viscosity syndrome, hypertrophic osteoarthropathy, and renal dysfunction. Treatment consists of supplemental oxygen therapy (if it increases arterial oxygen saturation), pulmonary vasodilator therapy, and iron supplementation when iron deficiency is present. Iron studies will allow you to diagnose and calculate the iron deficit for the appropriate replacement if indicated.
For many years, cerebrovascular complications were presumed to be due to erythrocytosis and phlebotomy was performed to maintain normal or near-normal hematocrit levels. However, a large study failed to demonstrate a relationship between hematocrit and stroke risk in cyanotic patients. Atrial fibrillation, systemic hypertension, and microcytosis, which can be worsened by phlebotomy and iron deficiency, are the strongest predictors of stroke in Eisenmenger patients. Phlebotomy, along with volume resuscitation and iron supplementation, may have a role in treating acute, severe symptoms of hyperviscosity, but is not indicated in this patient with mild symptoms (headaches, leg cramps, scotoma) that may be due to iron deficiency rather than hyperviscosity.
Hydroxyurea is used to suppress myeloproliferative activity (i.e., overproduction of all cell lines) in patients with polycythemia vera. In contradistinction to polycythemia patients, cyanotic patients have high levels of erythrocytes in proportion to their hypoxemia, but often have low platelet levels.
Suppression of bone marrow activity would not be appropriate in patients with secondary erythropoiesis. Eisenmenger syndrome is associated with both thrombocytopenia and thromasthenia as well as abnormal coagulation parameters, so that patients are at risk for both thrombotic events and bleeding events. Systemic anticoagulation or antiplatelet therapies are generally reserved for specific indications (clinically apparent thrombosis, atrial fibrillation, the presence of artificial heart valves, or conduits).
Uric acid levels are often elevated in Eisenmenger patients and are associated with worse outcomes.