What's the formula of the substance that should a student apply to the skin if he or she gets splashed with an acid?
H2O!
Elements in the same group are similar because
A. They have the same number of neutrons.
B. They have the same number of valence electrons.
C. They will form ionic bonds with water molecules.
D. They will form two ionic bonds with members from the alkali metals.
B. They have the same number of valence electrons.
What's the difference between a compound and a mixture?
Compounds are substances which can be formed by chemically combining two or more elements. Mixtures are substances that are formed by physically mixing two or more substances.
What's the formula for sulfuric acid?
A.H2SO3
B.H2SO4
C.H2S
D.HNO3
A.H2SO3
How does the concept of conservation of mass apply to chemical reactions?
A. The change in the amount of matter is only a small percentage of the total mass.
B.The reactants and products have exactly the same molecules.
C.The change in the amount of matter is equal to the change in energy.
D. The reactants and products have exactly the same atoms.
D. The reactants and products have exactly the same atoms.
Name and describe the 4 major types of reactions
:max_bytes(150000):strip_icc()/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
A solution of a base differs from a solution of an acid in that the solution of a base —
A. has a greater [OH-].
B. is able to conduct electricity.
C. has a greater [H3O+].
D.is able to change the color of an indicator.
A. has a greater [OH-].
Which of these is the best way to dispose of a potentially dangerous compound?
A. pour it down the drain
B. put it into a garbage can
C. recycle the compound for a future lab activity
D. place it in a hazardous material waste container
D. place it in a hazardous material waste container
The experiment shows a —
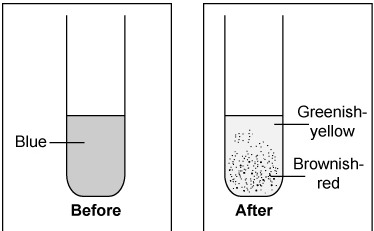
A. chemical reaction, because the solution changed color
B. physical reaction, because the solution changed color
C. physical reaction, because it involved a compound
D. chemical reaction, because it involved a mixture
A. chemical reaction, because the solution changed color
Soda contains water and sugar. Soda is —
A. a compound because its parts can only be separated through chemical means
B. an element because it is a pure substance whose parts cannot be separated
C. a mixture because its parts can be separated through physical means
D. a precipitate because it leaves a substance behind when it is heated
C. a mixture because its parts can be separated through physical means
In an ionic bond, a metal gives its e- away to a non-metal; whereas in a covalent bond, two non-metals share a pair of electrons.
The reaction between sodium and chlorine that forms table salt is shown.
__Na(s)+ Cl2(g)→__NaCl(s)
What coefficient should be added to the blanks to balance the equation?
2
Select all of the examples that show a decomposition reaction.
A.CO2→C+O2
B.Cu+O2→CuO2
C.Na2CO3→Na2O+CO2
D.Zn+CuSO4→ ZnSO4 + Cu
E.NaOH+HCl→NaCl+H2O
A.CO2→C+O2
C.Na2CO3→Na2O+CO2
What are the relative ion concentrations in an acid solution?
A. H+ ions but no OH- ions
B. More H+ ions than OH- ions
C. An equal number of H+ ions and OH- ions
D. Fewer H+ ions than OH- ions
A. More H+ ions than OH- ions
Which diagram below BEST illustrates how atoms are distributed in containers in gaseous and liquid states?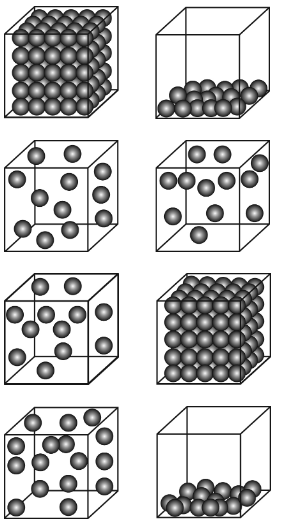
Which two elements have the most similar chemical properties?
A. Na and P
B. Be and Mg
C. Ca and Br
D. Cl and Ar
B. Be and Mg
After the addition of the iron, the test tube now contains iron (II) sulfate solution and copper particles. The iron has become part of a — compound or mixture?

compound with the sulfate, which must be separated through chemical method
What is the IUPAC name for the compound FeS?
A. Iron(III) sulfate
B. Iron(III) sulfide
C. Iron(II) sulfate
D. Iron(II) sulfide
D. Iron(II) sulfide
Select all of the examples that show a synthesis reaction.
A.2HCl + Zn → ZnCl2+H2
B. 2Al+ 3Br2 → 2AlBr3
C. 2H2O → 2H2+O2
D. Cu+O2 → CuO2
E. NaCl → Na + Cl
B. 2Al+ 3Br2 → 2AlBr3
D. Cu+O2 → CuO2
1 x 10-3
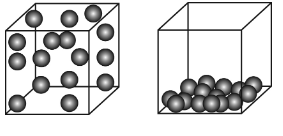
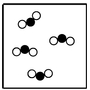
Which particle model diagram represents only one compound composed of elements X and Z?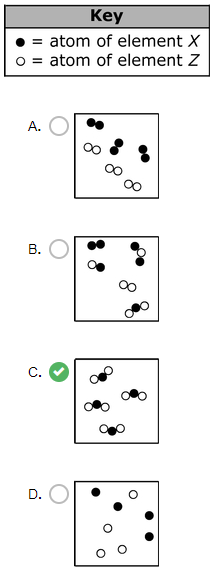
Which of the following descriptions includes an extensive property?
A. yellow in color, malleable, with a mass of 5 grams
B. red in color, ductile, with a melting point of 1100°C
C. colorless, odorless, with a freezing point of 0°C
D. high luster, malleable, and ductile
A. yellow in color, malleable, with a mass of 5 grams
What is the pressure of a mixture of CO2, SO2, and H2O gases, if each gas has a partial pressure of 25 kPa?
A. 25 kPa
B. 50 kPa
C. 75 kPa
D. 101 kPa
B. 50 kPa
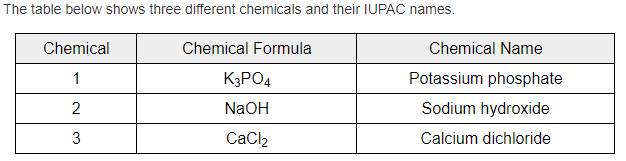
Which chemical has an incorrect IUPAC name?
Chemical 3
Double or nothing: Explain why???
Select all of the single replacement reactions.
A. Ca(OH)2→CaO+H2O
B. Zn+2HCl→ ZnCl2+H2
C.6CO2+6H2O→ C6H12O6 + 6O2
D. Fe+ Cu(NO3)2→ Cu + Fe(NO3)2
E. H2SO4+2NaOH→Na2SO4+2H2O
B. Zn+2HCl→ ZnCl2+H2
D. Fe+ Cu(NO3)2→ Cu + Fe(NO3)2
What is the pH of a 0.001 M HNO3 solution?
3
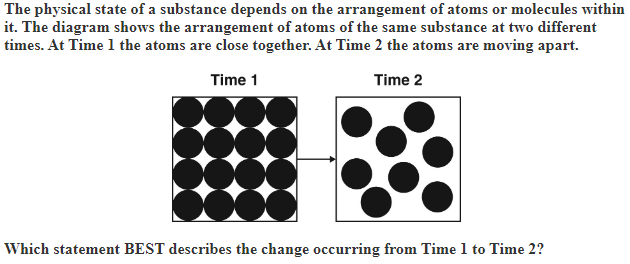
A gas becoming a solid.
B.A gas becoming a liquid.
C.A solid becoming a liquid.
D.A liquid becoming a solid.
C. A solid becoming a liquid.
Which of the following is an intensive property of a sample of water?
A. Mass
B. Molar mass
C. Number of moles
D. Volume
B. Molar mass
Gases X, Y, and Z, in a closed system at constant temperature, have a total pressure of 80 kPa. The partial pressure of each gas is shown below.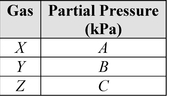
What is the partial pressure, in kPa, of gas X?
A. 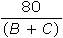
B.(B + C) − 80
C.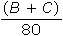
D.80 − (B + C)
D.80 − (B + C)
Fill in the blanks below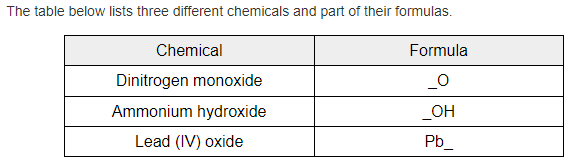
N2, NH4, O2
Select all of the double replacement reactions.
A. CaO+CO2→CaCO3
B. Na2CO3→Na2O+CO2
C. KCl+AgNO3→AgCl+KNO3
D. Cu+Ag(NO3)2→Ag+Cu(NO3)2
E. H2SO4+2NaOH→Na2SO4+2H2O
C. KCl+AgNO3→AgCl+KNO3
E. H2SO4+2NaOH→Na2SO4+2H2O
The [H3O+] of a solution is 1 × 10-8. This solution has a pH of —
A. 8, which is basic.
B. 6, which is basic.
C. 6, which is acidic.
D. 8, which is acidic
A. 8, which is basic.