Eficiencia
What are the horizontal rows on the periodic table called ?
Periods
The simplest form of matter is the
Atom
He created the Periodic Table
Mendeleev
Define the following terms:
Fission
Fusion
fission - Splitting of a nucleus
Fusion - joining of 2 nuclei
What is an Ionic Bond?
A bond where electrons are transferred between a metal and a nonmetal.
Reactions that release energy
Exothermic
What is atomic mass ?
total weight of protons and neutrons
What does this diagram represent
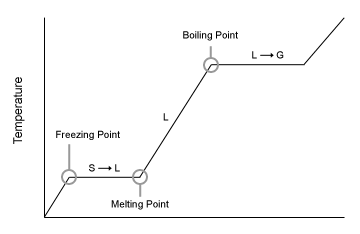
A heating Curve
What did J.J Thompson do ?
used the cathode ray tube to discover electrons
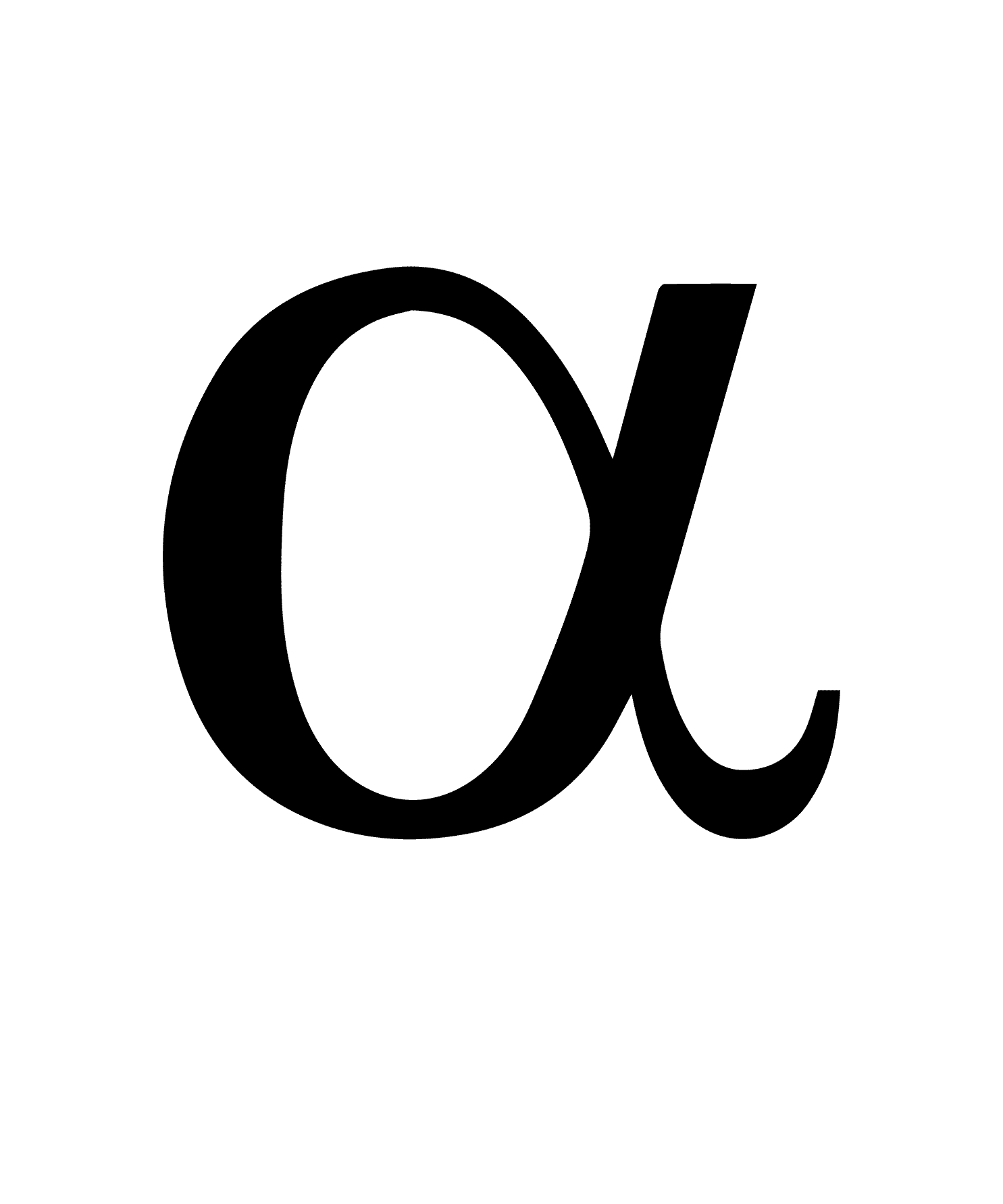 what does this symbol represent
what does this symbol represent
Alpha particle or Alpha decay
A diagram representing the Valence electrons of an element or compound
Lewis dot structure
When elements or molecules switch places
AC + BD ----->AB + CD
Replacement reaction
What is Bohr's Diagram ?
a diagram of an atom which includes all protons, neutrons, electron shells, and electrons
When a substance is changing from a solid to a liquid, what type of energy exists?
What is this transition called?
Potential energy exists when a substance is changing from a S- L.
This transition is called a phase change.
He was known for his gold foil experiment- fired negative ions at thin sheet of gold foil, discovered the atomic nucleus and proposed a nuclear model of the atom , who is he ?
Ernest Rutherford
An atom with 8 protons, 8 neutrons, and 8 electrons is an atom of this element.
Oxygen
What type of bond is produced by sharing electrons?
Covalent bonds
Anything that has mass and volume
Matter
What is an element ?
A molecule composed of one kind of atom; cannot be broken into simpler units by chemical reactions.
Give an example of an element and a compound
Is Water Heterogeneous or Homogeneous?
Are Fruit loops Heterogeneous or Homogeneous?
Element = Carbon.....
Compound = H2O
Water = homogeneous
Fruit loops = Hetereogenous
Thompson's model was called ?
The Plum Pudding Model
33. Radium-226 decays by alpha decay to
A) barium-131. B) cobalt-60.
C) radon-222. D) polonium-218.
C
What is electronegativity? What type elements will have low electronegativity? WHat type of elements will have a high electronegativity?
The desire to gain electrons. Metals have low electronegativity and non metals have High electronegativity.
A substance present before a chemical change
Reactant
What are the elements in Group 18 called ?
Noble Gases
What are the 3 subatomic particles and where are they located?
Neutrons - inside the nucleus
Electrons - outside the nucleus
Protons - Inside the nucleus
Who Was Democritus?
Greek philosopher that said all matter is made of tiny, indestructible particles called atoms
46. Sodium-24 has a half-life of 15 hours. How many hours is three half-lives?
A) 60 hours B) 45 hours
C) 30 hours D) 15 hours
E) 7.5 hour
B
What are 2 ways to identify a bond as ionic or covalent?
1. Determine types of elements that are bonding
m + nm = ionic
nm +nm = covalent
Electronegativity Difference greater than 1.7 = ionic
less than 1.7 = covalent
The product of a neutralization reaction.
Salt