Chlorofluorocarbons (CFCs) are gases that were once widely used as refrigerants and
propellants. When it was discovered that these molecules contributed to the depletion of
the ozone layer, their use was banned worldwide by 1987. However, we are still seeing their environmental contamination to this day.
Draw all constitutional isomers of a CFC with the molecular formula C2Cl3F3.
Carbons are tetravalent while halogens are monovalent therefore the two carbon atoms must be in the center (not Cl or F) and as a result there are only two possible isomers.

Draw the constitutional isomer of the compound below that you expect to have 1012 times more acidic. 
 Carboxylic acids typically have pKa around 4-5 while the starting molecule has a pKa around range of 16-18 which satisfies the criteria of being 1012 times more acidic.
Carboxylic acids typically have pKa around 4-5 while the starting molecule has a pKa around range of 16-18 which satisfies the criteria of being 1012 times more acidic.
Draw the enantiomer
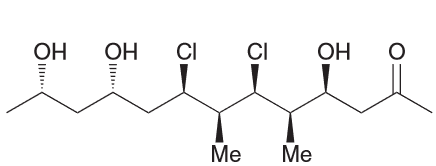
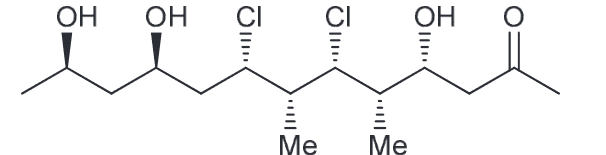
Predict the major product.
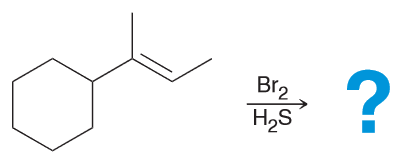
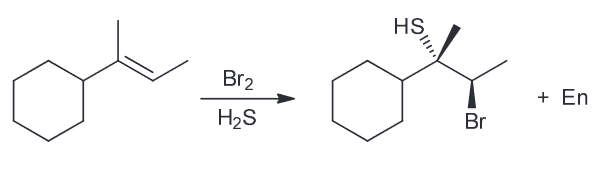
Lithium salts have been used for decades to treat mental illnesses, including depression and bipolar disorder. Although the treatment is effective, researchers are still trying to determine the mechanism of action that lithium salts undergo as mood stabilizers. Draw the lewis structure of the uncharged Li and of the Li+.
Li is in G1A which means it has only 1 valence electron, the loss of that electron to form the cation means that there will be no valence electrons drawn for the second structure.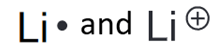
There are 19 constitutional isomers with the molecular formula of C8H18. Without drawing all 18 isomers, determine how many of them will have the parent chain being heptane.
Only 3 consitutional isomers that would have heptane as the parent chain.
Since there are 8 carbons in the compound, that means there is one (most likely CH3) that can be placed on various positions. It can't be placed on the end because that would make the parent octane. You can however put that CH3 either at C2, C3, or C4 location to yield the following constitutional isomers:
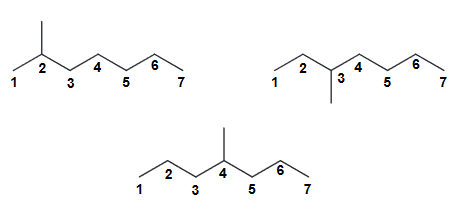
If you place it at C5 or C6 then you will regenerate the C2 or the C3 position.
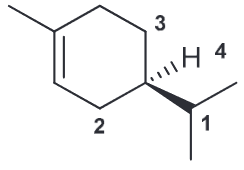
Identify whether this is (R) or (S)
R
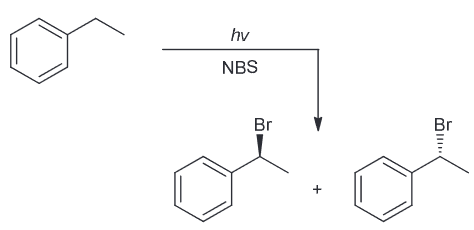
When ethylbenzene is treated with 1 eq of NBS and irradiated with UV light, there are two stereoisomeric compounds obtained in equal amounts. Draw the products and explain why.
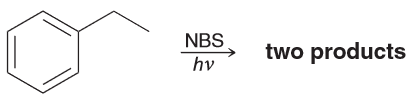
The expected benzylic radical can be attacked from either above or below which will result in a racemic mixture of enantiomers.
Methylene chloride (CH2Cl2) has fewer chlorine atoms than chloroform (CHCl3). Nevertheless, methylene chloride has a larger molecular dipole moment than chloroform. Explain
The third Cl atom in CHCl3 partially cancels the effect of the one of the other two atoms which reduces the molecular dipole compared to CH2Cl2.
What is the strongest base present after CH3MgBr is treated with water. (Hint: Remember that metals tend to be spectator ions, meaning they do not participate in the reaction and only are there to help stabilize the charge.)
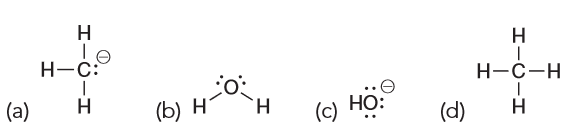
Answer is C
The difference in electronegativity between C and Mg is so great it is usually drawn as an ionic bond. Metals always have a positive charge which means the negative charge is on the carbon, forming a carbanion. However, the reaction is in water which means we have a levelling effect which means that despite -:CH3 normally one of the strongest bases, in water nothing stronger than hydroxide can be produced.
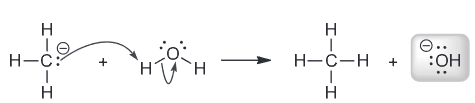
Which of the following can be used to form 1-butene.
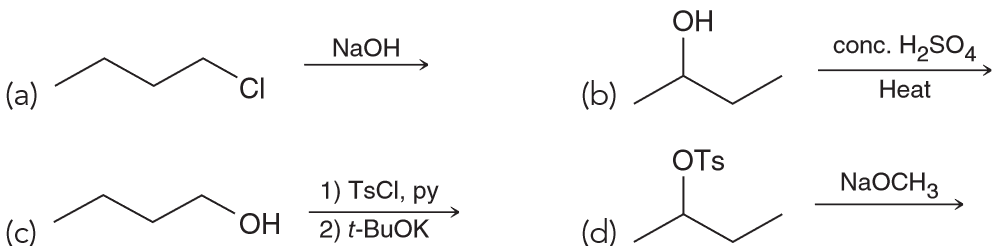
Answer choice C
A involves a primary substrate being treated with a strong base and the SN2 product should dominate over E2. B and D would produce a substituted alkene, not 1-butene.
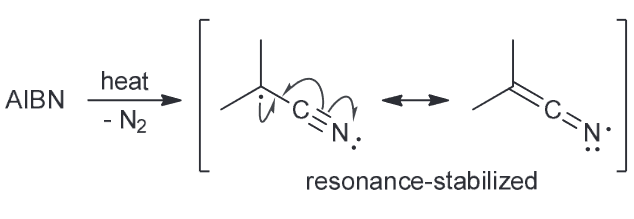
AIBN is an azo compound that is often used as a radical initiator. Upon heating it will release N2 to produce two identical radicals. Explain why these two radicals are so stable.
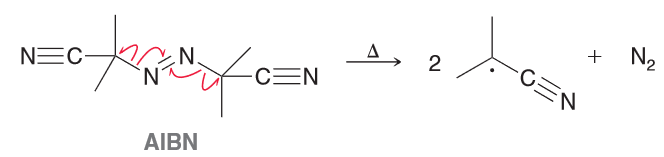
heating will generate a resonance stabilized radical. In addition to that the presence of methyl groups (electron donating groups) will further stabilize the unpaired electron.
Which compounds are constitutional isomers?
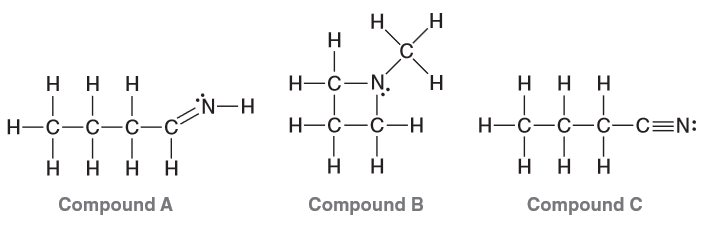
Compounds A and B both have the formula C4H9N but have different connectivity which means that they are the constitutional isomers.
The anti conformation of ethylene glycol is not the lowest energy con-
formation. Explain why the other two staggered conformations are actually lower in energy than the anti conformation.
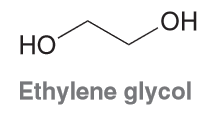
Hydrogen Bonding
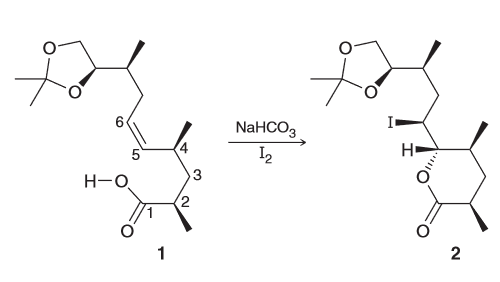
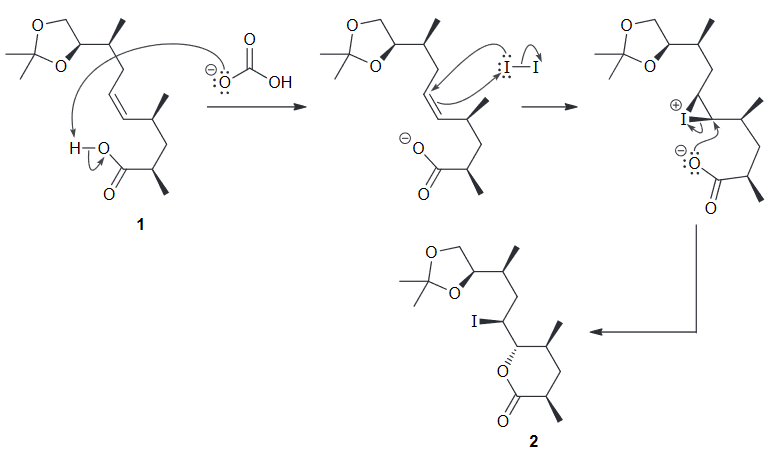
A potent antibiotic can be diastereoselective when reacted with NaHCO3. Draw the newman project for the compound below when looking down the C4-C5 bond and use this to rationalize the observed diasteroselectivity.
First simplify the drawing by assigning the two larger groups as R and R'
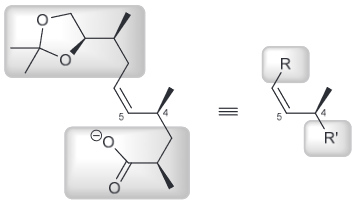
Then you can look down the C4-C5 bond and make a newman project. To better see the top and bottom face of the pi bond but not change the conformation we can rotate it by 90 degrees.
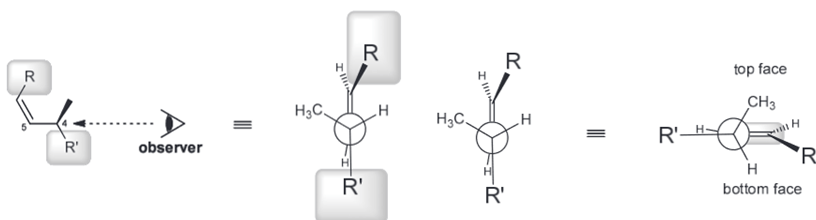
Notice that the two largest groups are farthest away from each other. Gauche interactions between Me and R, and between R' and H are observed. We can avoid the first interaction by rotating counter clockwise.
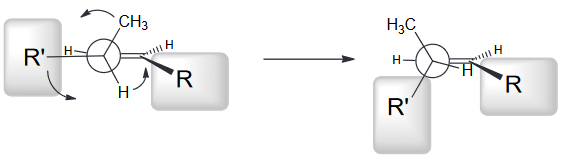
From this we see that the top face will be more accessible most of the time and will as a result be lower in energy and thus more favorable.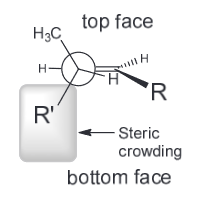
When isopropylbenzene is treated with UV light and 1 eq of NBS, there is only 1 product obtained. Explain why.
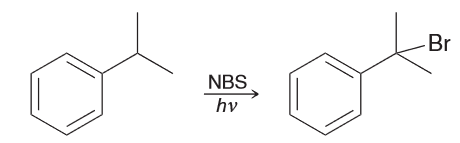
The benzylic hydrogen atom is the only one that can be abstracted to generate a resonance stablized radical which means that the benzylic position is selectively brominated.
Identify the functional group highlighted in the compound below. This functional group is highly versatile and enables the preparation of copious new compounds and natural products.
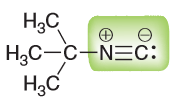
Once you determined the functional group, identify the hybridization for each atom highlighted. What atomic orbital does the carbon containing the lone pair (LP) occupy?
Functional Group is nitriles- specifically this is an isonitrile. Hybridization is sp for both N and C (due to the triple bond, steric number 2 since 2 things are attached to it and no LP on N and one thing attached to C along with 1 LP); this also means that the LP resides in the sp hybridized orbital.
Treating a lactone (cyclic ester) with sodium hydroxide will produce an anion. Draw the product of the intramolecular proton transfer for this acid-base reaction and identify which side is favored by equilibrium and why.
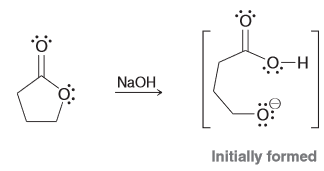
Equilibrium favors the weaker base/acid which means that it will favor the product versus the starting.
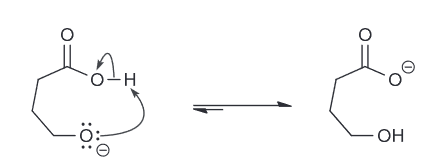
What is the major product for this reaction
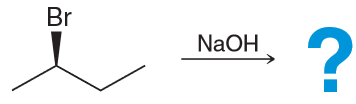
reagent is both a strong base and strong nucleophile which means that it will favor either SN2 or E2. However the substrate is a secondary substrate which means it will favor E2 as the major. We also know E2 is stereoselective which means we expect the trans- isomer.
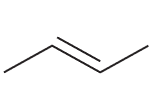
Explain why the compound below would not be a good radical initiator
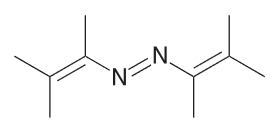
Loss of N2 would result in the formation of a vinylic radical which is too unstable to form.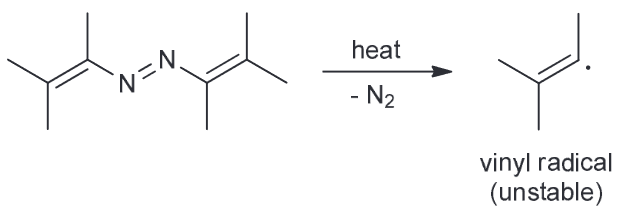
One of the products produced from isonitriles is a isonitrile dihalide, shown below. Looking at the highlighted atoms, what orbital does the lone pair (LP) reside in on the N and what is the hybridization? What are the C-N-C bond angles?
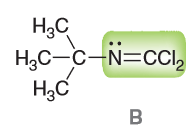
LP resides in sp2 hybridized orbital (N has a steric number of 3, two things attached and 1 LP which means that the hybridization is also sp2 for N). C has a steric number of 3 which means it is also sp2 and each Cl has a steric number of 4 indicating an sp3 hybridization. Because C and N are both sp2 that means the bond angle is expected to be approximately 120°
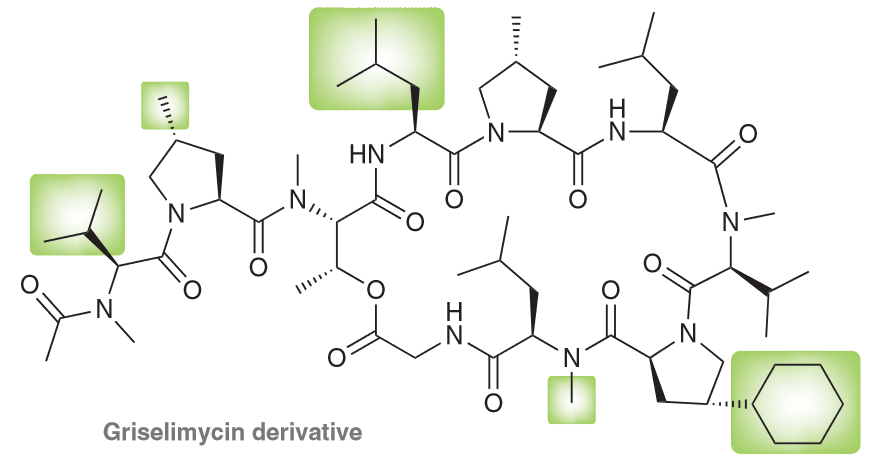
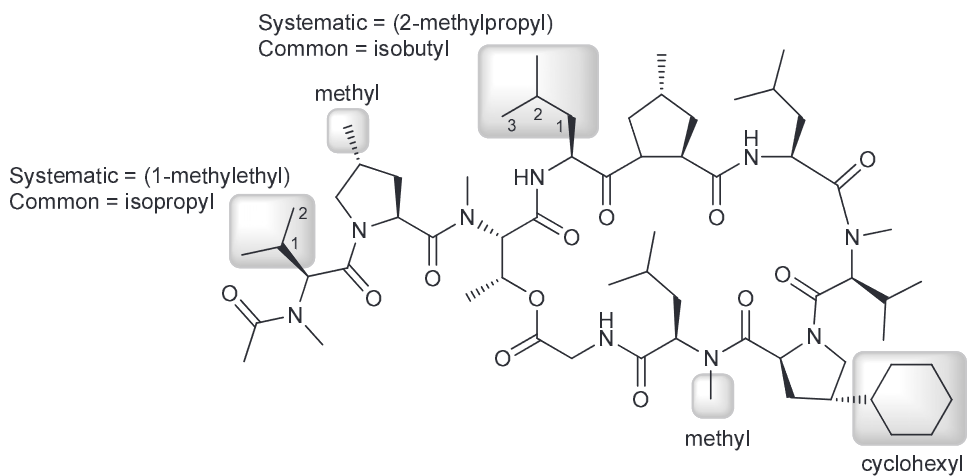
Indicate the systematic name and the common name for all the highlighted substituents
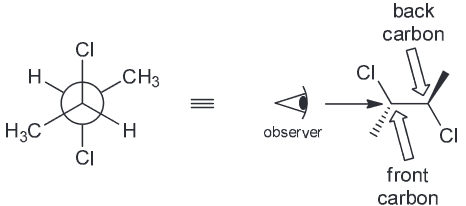
The compound below has a plane of symmetry - find this plane of symmetry. (Hint: You may need to rotate some bonds)
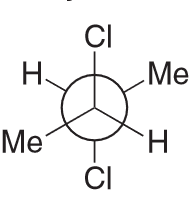
There are two ways we can do this:
1) You can identify the plane of symmetry using just the newman projection by rotating the central C-C bond 180 degrees and see that the 2 Cl atoms are eclipsing each other and so are the methyl groups and hydrogens
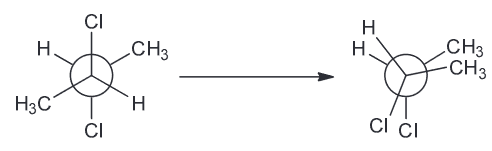
As a result we can clearly see that there is a plane of symmetry (between C2-C3)
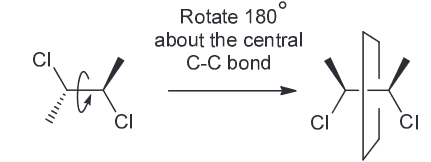
2) First convert the Newman projection into the bond-line.
Then rotate 180 degrees
Propose an efficient synthesis
Cycloserine is an antibiotic isolated from the microbe Streptomyces
orchidaceous. It is used in conjunction with other drugs for the treatment of tuberculosis. Based on the structure below, identify the molecular formula and draw all significant resonance structures.
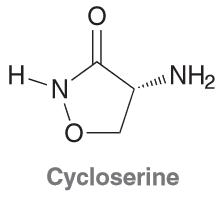
MF: C3H6N2O2
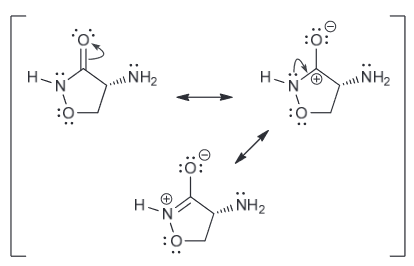
Resonance structures:
Predict which compound is more acidic and explain why.
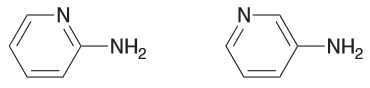
Both are resonance stabilized but the first one has the negative charge spread over two N and a C.
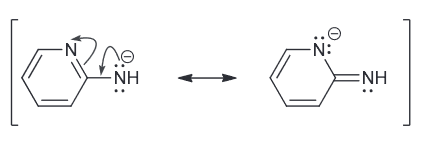
While the second one has a negative charge spread over just one N and one C.
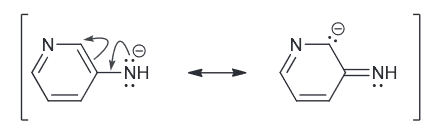
Since N is more electronegative atom and is therefore more capable of stabilizing the negative charge and therefore the first compound is more acidic.
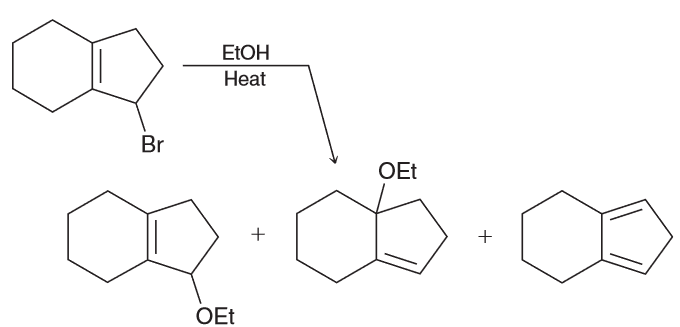
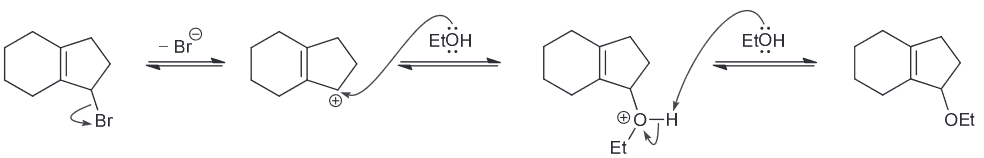
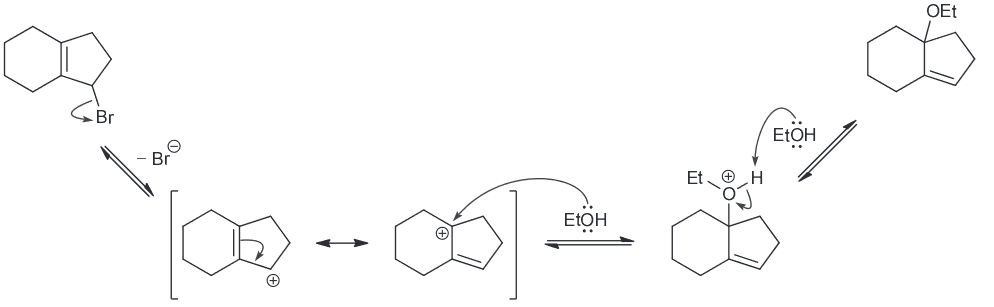
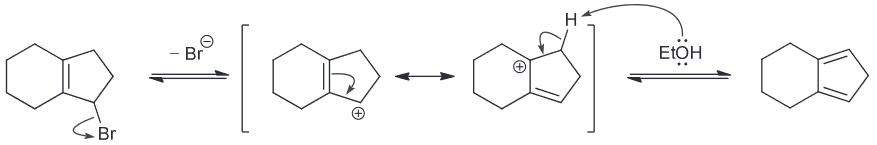
When butyl bromide is treated with sodium iodide in ethanol the concentration of iodide quickly decreases, but then slowly returns to its original concentration. Identify the major product of the reaction.
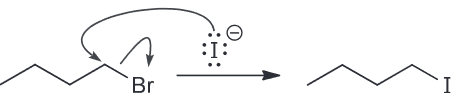 I is a much stronger nucleophile than EtOH so we expect it to attack the primary substrate in an SN2 reaction to generate a butyl iodide.
I is a much stronger nucleophile than EtOH so we expect it to attack the primary substrate in an SN2 reaction to generate a butyl iodide.
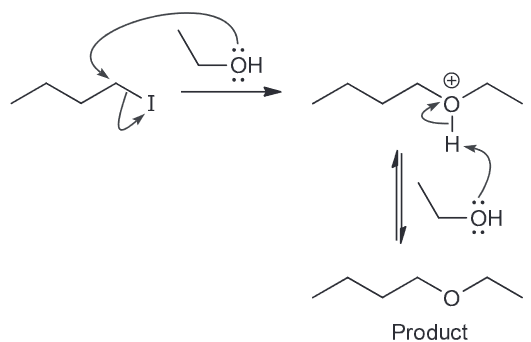
As a result the concentration of I decreases pretty quickly however also remember that I is also a good LG. This means there is another nucleophile (the weaker EtOH) that will be slowly ejecting the I ions. A slow SN2 reaction will occur with the resulting oxonium ions being deprotonoated (be other EtOH which will function as a base) to form an ether as the major product.
There are 3 constitutional isomers with the molecular formula of C5H12. Chlorination of one of these isomers yields only one product. Identify the isomer and draw the product of chlorination.
There are three isomers possible with that molecular formula
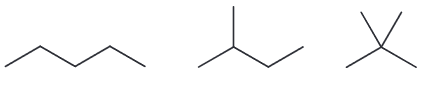
Chlorination of the first gives 3 isomeric chloroalkanes (1-chloro, 2-chloro, and 3-chloropentane) while monochlorination of the second compound will give for constitutionally isomeric chloroalkanes. The last one, however, will only yield one product.
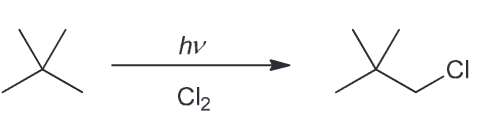
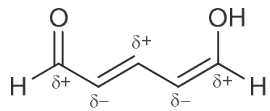
In the compound below, identify all carbon atoms that are electron deficient (δ+) and all carbon atoms that are electron rich (δ−). Justify your answer with resonance structures.
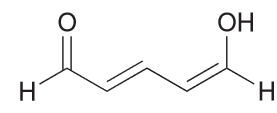
Resonance structures help identify electron rich and electron deficient atoms therefore I recommend drawing that first.
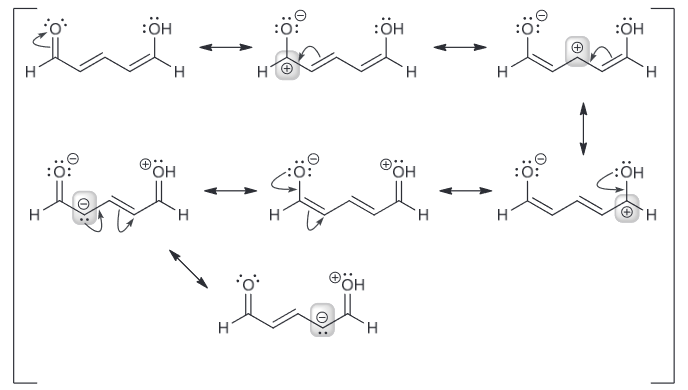 Based on the resonance structure a position that bears a positive charge is δ+ and those that have a negative charge δ-.
Based on the resonance structure a position that bears a positive charge is δ+ and those that have a negative charge δ-.
All of the following represent cis-1,2,-dimethylcyclohexane except
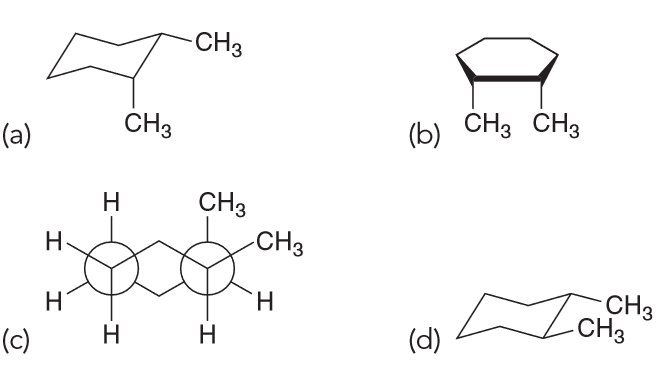
D because it is trans-1,2-dimethylcyclohexane
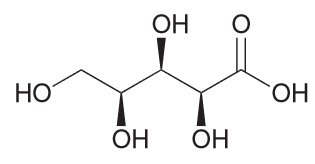
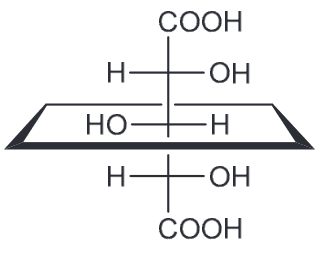
Draw the fischer projection for the following compound, being sure to place the -CO2H group at the top.
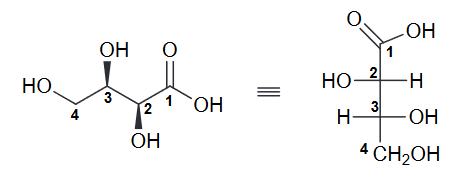
Explain why each of the following ROHs cannot be prepared via hydroboration-oxidation? What reaction would generate these products if possible?
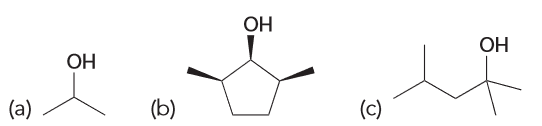
A) if 1-propene is the starting alkene, then the OH wouldn't be installed on the appropriate carbon. This reaction that would generate it would be acid-catalyzed hydration.
B) hydroboration-oxidation would give a syn addition across the double bond. However it would be the syn addition of OH and H. A substitution reaction could yield this but the solvent used would determine which type.
C) no starting alkene would ever yield the desired product because it would need to be anti-markovnikov. Substitution (SN1) reaction could generate this product.
Warfarin, a courmarin derivative, is used as a blood thinner to prevent potentially fatal blood clots. Using the schematic below, how might you used IR spectroscopy to monitor the conversion of compound 1 into compound 2. Describe at least three different signals you can analyze to confirm the transformation.
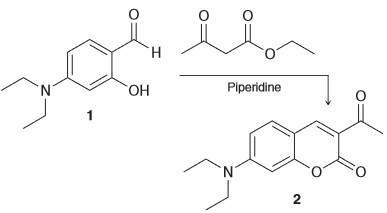
Compound 1 has an OH group which has a signal of 3200-3600 cm-1 and this can be one signal to distinguish that transformation since compound 2.
Compound 1 also has an aldehyde group which will produce a C-H signal around 2750-2850 cm-1 (also isn't not present in compound 2 and therefore a good signal to distinguish between the two).
Furthermore compound 1 only has one C=O bond while compound 2 has two C=O which means that the signal around 1680 cm-1 will be present in both for the ketone but compound 2 will also have a C=O peak for the ester (1700 cm-1).
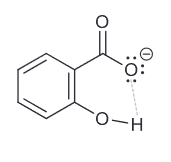
Salicyclic acid is more acidic than it's constitutional isomer para-hydroxyybenzoic acid. Explain why this is the case.
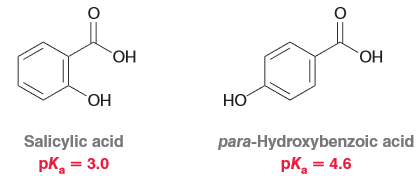
Salicylic acid has the inductive effect of the OH group being closer to the deprotonation site. Furthermore it can exhibit intramolecular hydrogen bonding which ultimately is the more contributing factor to it's stronger acidity (remember HB is one of the strongest molecular forces).
Suggest an mechanism of formation for the epoxide below when presented with a strong base.
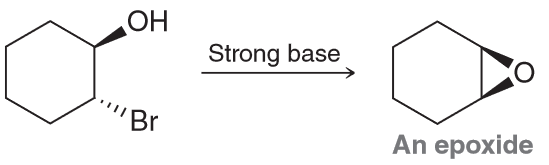
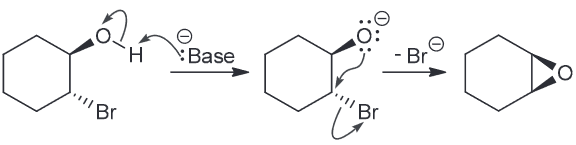
A strong base will remove the most acidic proton (the one on the ROH), producing an compound with both a nucleophilic and electrophilic center which will allow for an intramolecular SN2 reaction.
Draw a mechanism that is consistent with the transformation below and explain why the Cl is on the less substituted carbon (so why is the less substituted carbocation intermediate more stable?)
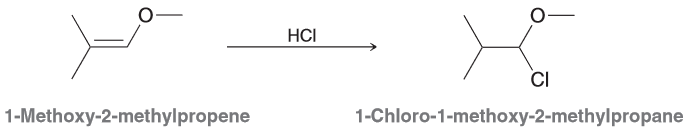
Mechanism should comprise of two steps: 1) proton transfer, 2) nuc attack. There are two possible regiochemical outcomes for the PT step, however one is favored more so because it is resonance stablized which is preferential even when compared to a tertiary carbocation.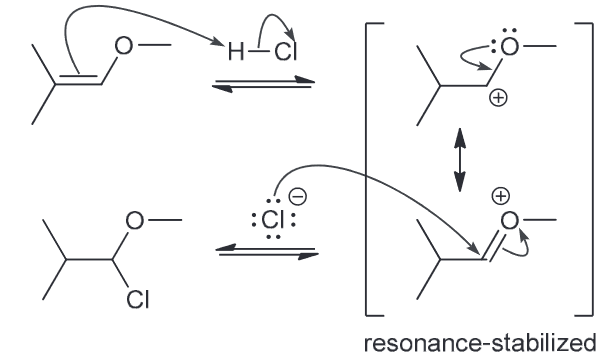
Describe how IR spectroscopy can be used to monitor the conversion of 3 to 4. Specifically, describe what you would look for to confirm complete hydrolysis of the acetate groups. 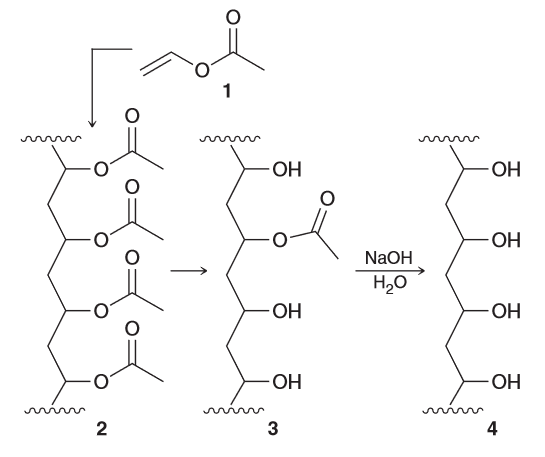
2 contains only ester groups, so the IR spectrum of 2 is expected to exhibit a signal near 1740 cm-1 (typical for esters), associated with vibrational excitation (stretching) of the C=O bond. 4 lacks any ester groups, so the signal near 1740 cm-1 is expected to be absent in the IR spectrum; instead it has OH groups, which are expected to produce a broad signal in the range 3200-3600 cm-1. 3 has both functional groups (alcohol group and ester group), so an IR spectrum of is expected to exhibit both characteristic signals. When 3 converts to 4 we expect the loss of the single around 1740 cm-1
Draw both chair conformations of this compound and explain which is more stable. (Hint: Begin drawing a numbering system and then determine the location and 3D orientation of each substitutent; the first number can be placed anywhere on the ring so long as the remaining numbers go clockwise)
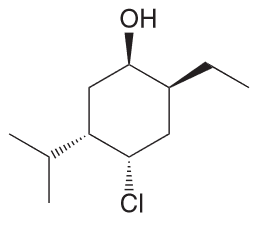
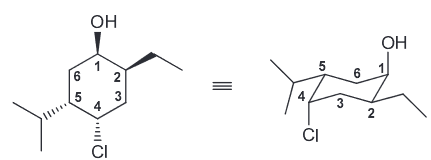 Once the first is drawn, use the numbering system again. Notice that the ring flip causes all equatorial groups to become axial and vice versa.
Once the first is drawn, use the numbering system again. Notice that the ring flip causes all equatorial groups to become axial and vice versa.
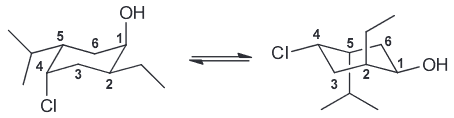
Draw the bond-line structures (with wedges and dashes) for these compounds:
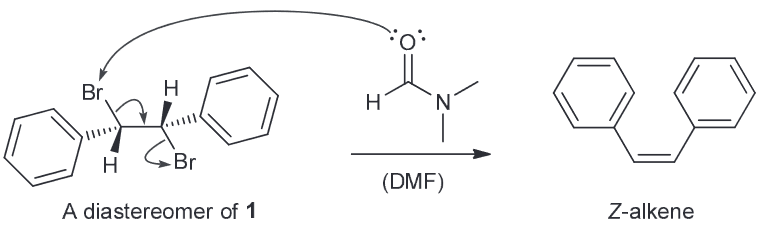
Compound 1 undergoes debromination upon treatment with DMF to afford an alkene. What type of isomer is compound 2?
The mechanism above has been refuted by the observation that diasteromers of compound 1 also afford the isomer when treated with DMF exclusively.
Explain why this mechanism is inconsistent with the observation. Is this reaction stereoselective or stereospecific - explain.
E-isomer.
The proposed mechanism is a concerted one that closely resembles an E2 process. As a result we would expect that the two bromines are anti-periplanar to one another for said elimination to occur. This means that we would actually generate the Z-isomer based on this mechanism.
Since the E-alkene is obtained from 1 and its diastereomers which means it does not have a requirement for anti-periplanarity, meaning it cannot be a concerted mechanism.
Because of the preference for the E-isomer isn't dependent on anti-periplanarity of the starting dibromide the reaction must be stereoselective since only one configuration of the alkene is favored.
Every compound with a molecular formula C5H11N has no π- bonds. Every carbon atom is connected to exactly two hydrogen atoms.
Determine the structure of the compound.
C is tetravalent and N is trivalent meaning that both can be considered center atoms while H atoms can only be terminal since they are monovalent. If we draw a linear or even branched skeleton with the 5 C and N atom we find that the number of H atoms are incorrect (more than the indicated). This indicates that we should look at pairing electrons as double or triple bonds or make the structure a ring to decrease the amount of hydrogens.
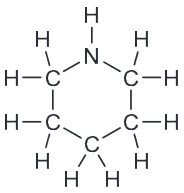
If we draw a ring we do get the correct number of hydrogens but the other criteria is not met, namely that each C is connected to exactly two H.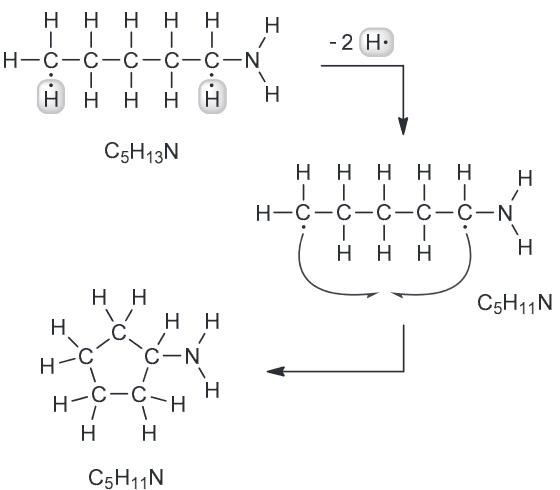 However there is an easy way to fix that - increase the size of the ring from 5- to 6-membered ring.
However there is an easy way to fix that - increase the size of the ring from 5- to 6-membered ring.
MARIO was a tool we taught to help you determine the factors for comparing relative acidity of compounds; when two of these factors are in competition the priority is covered generally in that ARIO order. However there were exceptions mentioned such as with C-triple bond version amines. Compare the two compounds below to determine if they also constitute another exception and justify your choice.
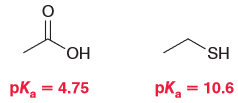
First look at the conjugate base of each compound
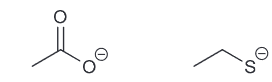
This means that there is an exception here since "resonance" is the dominant factor than the "atom"
Draw a plausible mechanism for the following ester being treated with LiI in DMF. Then explain why the reaction would occur ten times faster when a methyl ester is used instead.
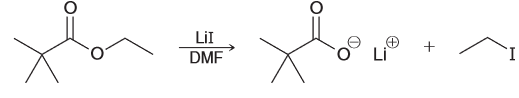
Nucleophile is I- and the solvent is polar aprotic indicating an SN2 reaction. The substrate is a primary substrate.
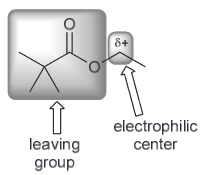
Therefore the mechanism would be as followed
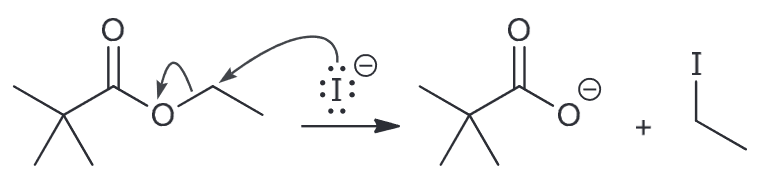
Remember that SN2 is highly sensitive to the nature of the substrate and it will proceed faster with the less sterically hindered ethyl ester.
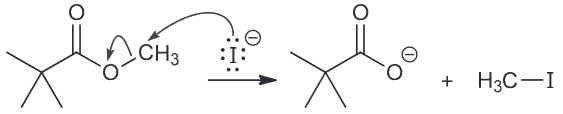
Propose a mechanism for the formation of each of the following products
CL-20 and HMX are both powerful explosives. CL-20 produces a
more powerful blast but is generally considered too shock-sensitive for
practical use. HMX is significantly less sensitive and is used as a standard military explosive. When a 2:1 mixture of the two compounds is cocrystallized, the resulting explosive is expected to be more powerful than HMX alone, but with a sensitivity similar to HMX.
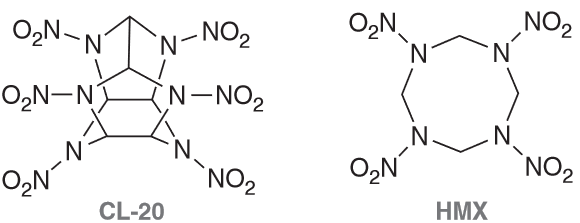
Identify the molecular formula for both explosives. Are the lone pairs on the N atoms localized or delocalized?
CL-20 MF: C6H6N12O12 and it's N LP are delocalized by resonance
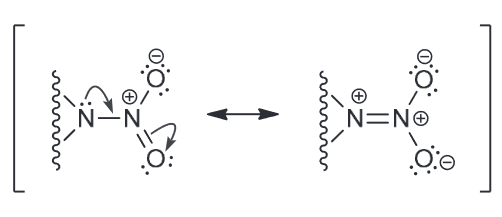
HMX MF: C4H8N8O8 and the LP on N are delocalized (they behave the same as CL-20 shown above since they are BOTH N-NO2 groups)
Which of the following is the constitutional isomer of heptane?
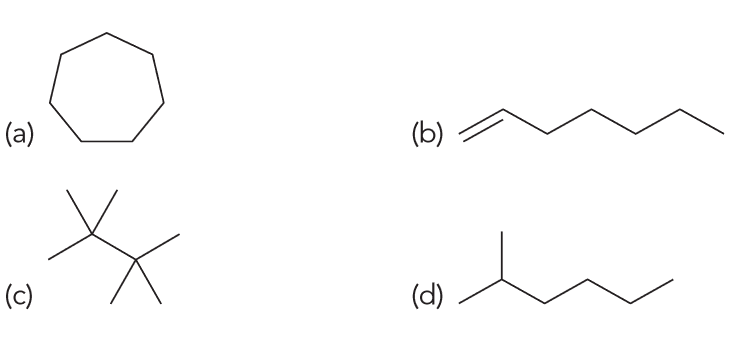
Heptane has the molecular formula C7H16, so we are looking for another compound with the same molecular formula.
A and B has the wrong number of hydrogens, C has the wrong number of carbons so D is the only possible answer.
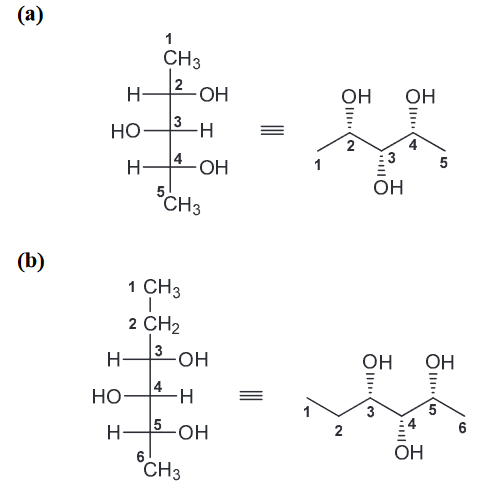
How many stereoisomers are expected for the following compound? Draw all of these stereoisomers.
There are 3 chirality centers (CC) so we would expect 8 stereoisomers, (23 = 8). But that is incorrect because the middle CC is not actually a CC (there are not two different groups attached). But for clarity we will draw the assumed initial 8 stereoisomers and label them as such.
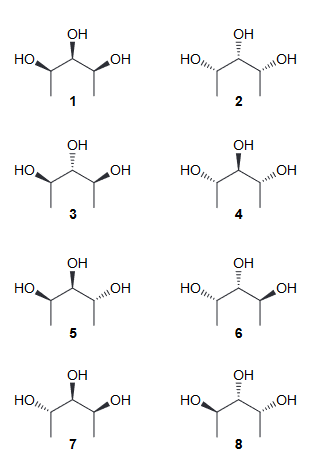
However when we look at them carefully, 1 and 2 represent one compound (meso) and so 3 and 4 represent another meso compound. Furthermore, 5 and 8 represent the same compound (flipped 180) and so do 6 and 7 (also flipped 180). Now when we look at the stereoisomers 5-8 we can notice that changing the configuration of the middle CC there is no stereoisomer produced. Therefore there are only 4 (1, 3, 5, and 6)
Draw the mechanism for this transformation
With spectroscopic techniques we chemist can determine the structure of an unknown compound; this techniques have been around for several decades and have been remarkably improved since it's initial development. Based on the spectroscopy you've been quizzed on, try to determine the structure of the unknown compound and, if there are any, draw the constitutional isomer of the compound with the following clues:
i) MF = C4H10N2
ii) no π-bonds present
iii) no net dipole
iv) exhibits strong hydrogen bonding
Consider the formula - C is tetravalent, N is trivalent and H is monovalent, this means that both C and N can be central atoms - play around with them as the central atoms only. H can only be on the ends and never as a central atom. Based on this knowledge there are two constitutional isomers possible.
Identify the most acidic proton is rilpivirine which is an anti-HIV drug.
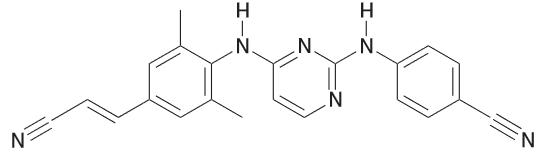
Ha is expected to be more acidic since it will produce a conjugate base (CB) that has one more resonance structure than Hb will produce. Furthermore, the CB of Ha can distribute the charge over 4 N and 5 C where the latter would spread it over 4 N and 4 C.
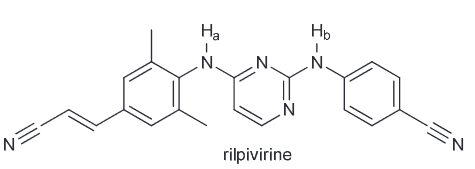
Provide the reagents that can produce this transformation. (Hint: Consider what is newly added, and if there are any stereocenters which are inverted).
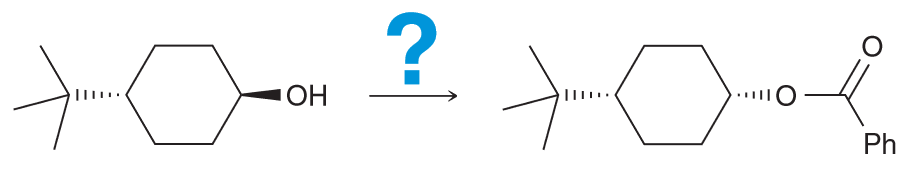
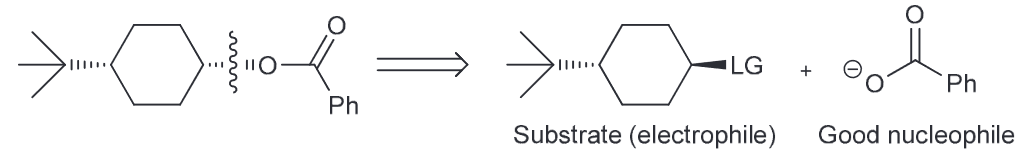
Notice that the ester group is new and that the CC there is inverted from the initial OH. We also notice that the OH is no longer present in the final product, so you must consider ways to successfully remove the OH. There are two ways - protonate with an acid and have water leave or TsCl, pyridine (py) to generate a good LG of OTs.
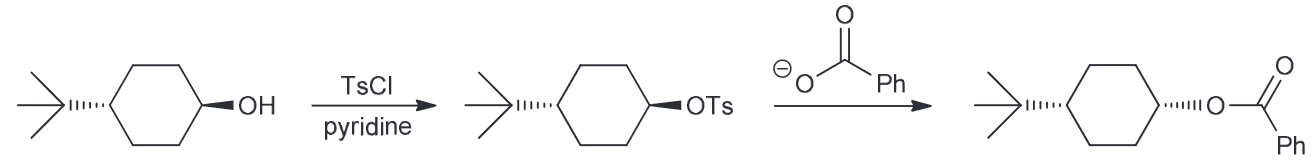
Now we need to consider what our new molecule looks like with it's LG. Notice how we have a secondary substrate (SN2 as a result especially since the nucleophile that we would need to use couldn't be a strong base since it would need to be resonance stabilized so we can generate our carboxylic acid on the product). There will be little competition with E2. As it is SN2 that means there will be a backside attack and thus the required inversion of configuration.
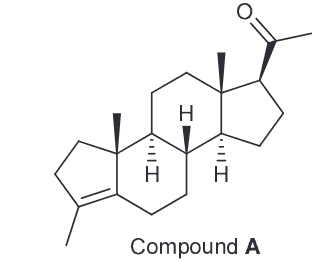
Determine the identity of compound A
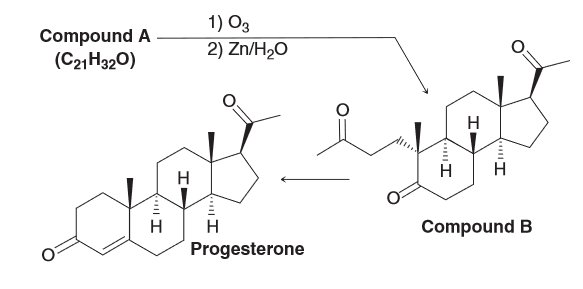 Theoretically there could be 3 different alkenes, however of those 3 only one is reasonable. Determine which one is the correct structure of A.
Theoretically there could be 3 different alkenes, however of those 3 only one is reasonable. Determine which one is the correct structure of A.
Answer:
When looking at the molecular formula we see that A contains the same number of carbon atoms as B (the ozonolysis product) so we can predict the structure of A by choosing two C=O groups and removing the oxygen atoms present and connecting the sp2 hybridized carbons with a double bond. Since there are 3 carbonyl groups that means there are 3 possible alkenes.
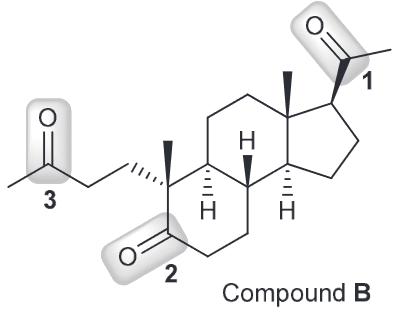
Attachment of carbons 1 and 2 would result in a heavily strained ring system that would not exist. Furthermore they are remotely located on opposite ends, pointing in opposite directions and would result in a very rigid, impractical structure. That means we can rule this alkene out.
Attachment of carbons 1 and 3 would yield similar results as above despite them being closer together and linked by a (slightly) more flexible carbon chain), but it would be a trans relationship resulting in far too much angle strain.
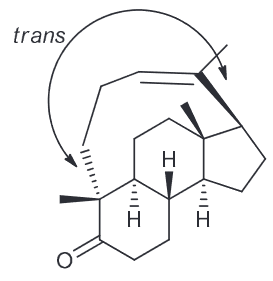
As a result the only possible alkene would be from the fused cyclopentene precursor that would occur when combining C2 and C3.
Which is the correct hybridization state and geometry for the
carbon atom in HCN?
linear, sp
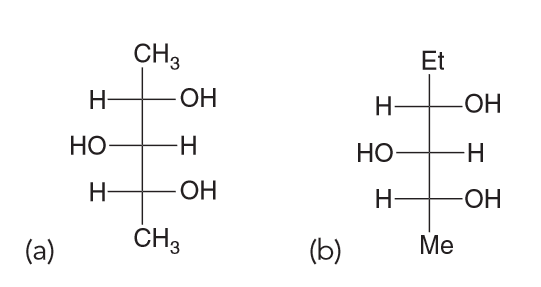
Draw all three staggered conformations for the following com-
pound, viewed along the C2−C3 bond. Determine which conformation is the most stable, taking into account gauche interactions and hydrogen-bonding interactions. Provide a reason for your choice by identifying all of the interactions that led to your decision.
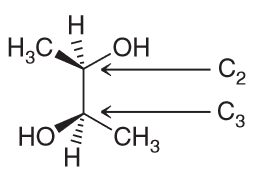
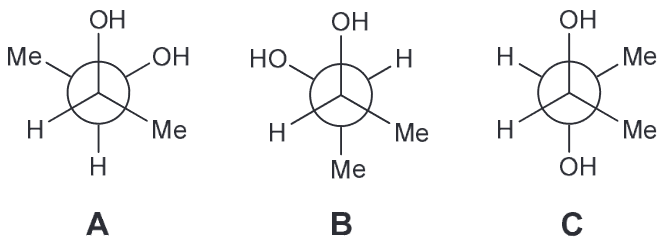
There are Me-Me, Me-OH and OH-OH. OH can have Hydrogen bonding interactions which will help stablize them more so than destablize. Me-Me interactions will be destablizing because of steric effects. Me-OH will have some steric effects but not as much as Me-Me.
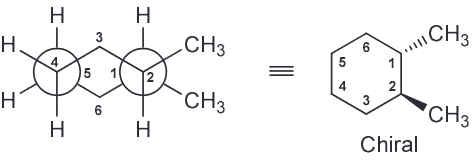
Based on all of this we expect the OH-OH interactions in A and B to be more favorable; C has them too far apart to have hydrogen bonding (HB) present. If we assume that the HB between OH-OH is the same in both A and B that means B should have the lowest energy because the next interactions present will be OH-H whereas A will have OH-CH3 interactions also present.Which compound is optically active
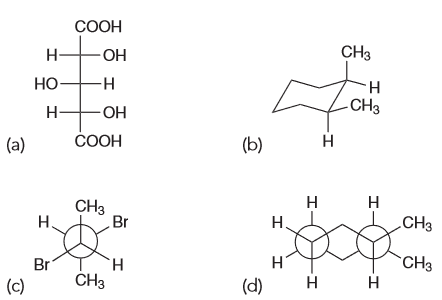
Compound A has a plane of symmetry and is therefore a meso compound and therefore optically inactive.
Compound B is racemic which means it is shown in the chiral conformation but it will equilibrates with its enantiomer; this means that it will be optically inactive.
Compound C is also a meso compound which can be seen if we rotate the central carbon by 180
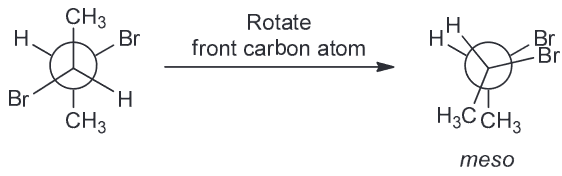
By process of elimination D has to be the correct answer and it is since the compound is chiral.
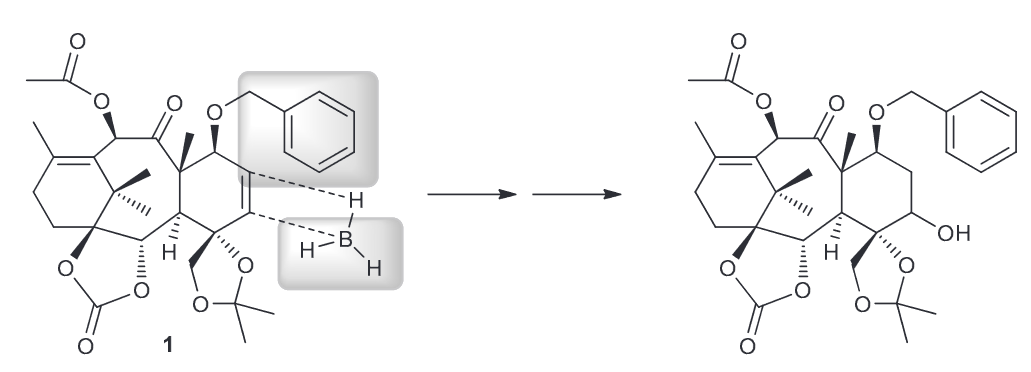
Identify the functional group in 1 that will readily react with BH3 and justify your choice and predict the regio- and stereochemical outcome for the process.
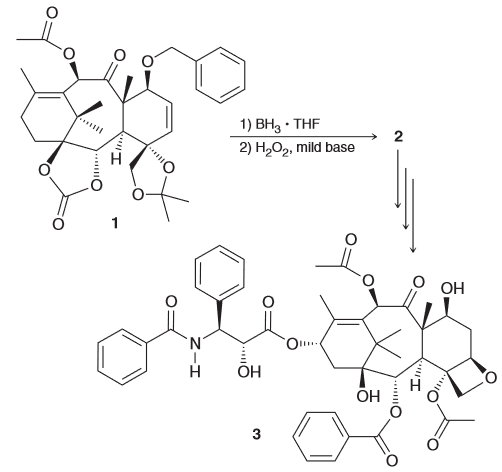
the functional groups that would react are the alkenes. Hydroboration is sensitive to factors which means the less substituted alkene will react more quickly. Notice how one side of the alkene will have a phenyl group interacting with the BH3 creating some steric hindrance on the front face, essentially blocking it. This means that a back-attack will be more favorable.
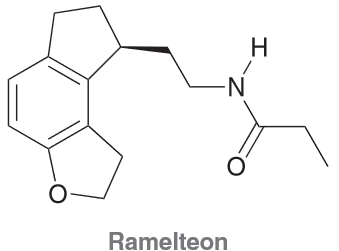
Ramelteon is a hypnotic agent used in the treatment of insomnia.
Based on the structure above identify the following
i) Molecular formula
ii) whether each lone pair (LP) are localized or delocalized
iii) the geometry of each atom (excluding H)
i) C16H21NO2
ii) All LP on O in C=O are localized. The ether LP and the N LP are delocalized via resonance.
iii) All sp2 hybridized carbon atoms are trigonal planar.
All sp3 hybridized carbon atoms are tetrahedral. The
nitrogen atom is trigonal planar. The oxygen atom of the
C=O bond does not have a geometry because it is connected to one other atom and the ether O has a bent geometry.
Explain why the addition of a cyano group can drastically decrease the pKa of RNH2 (Hint: what isi the functional groups being asked about and what are the initial pKas?)
RNH2 is an amine which exhibit pKas around 35-45. Cyano groups are CN group with a triple bond between them.
 Due to the triple bond present and the addition of a C and N, the cyano group allows for resonance stabilization which was not present in the amine functional group.
Due to the triple bond present and the addition of a C and N, the cyano group allows for resonance stabilization which was not present in the amine functional group.
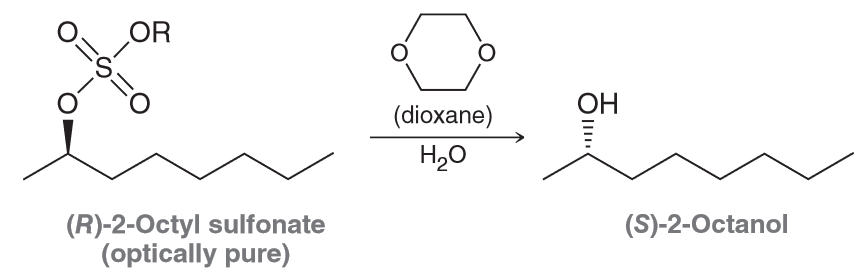
Draw the complete mechanism for the formation of (R)-2-octanol in the presence of dioxane via two successive SN2 reactions with dioxane functioning as the nucleophile.
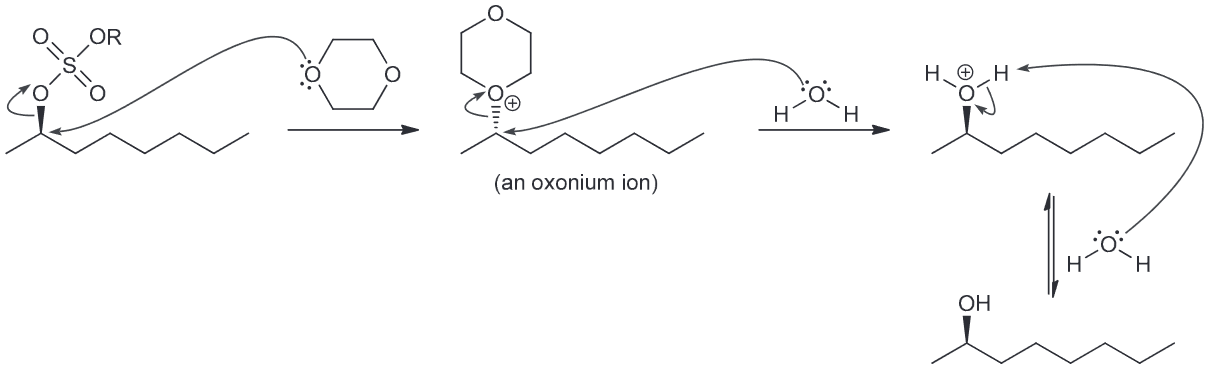
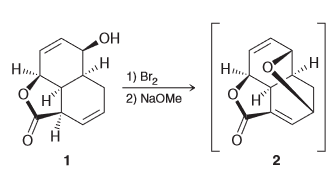
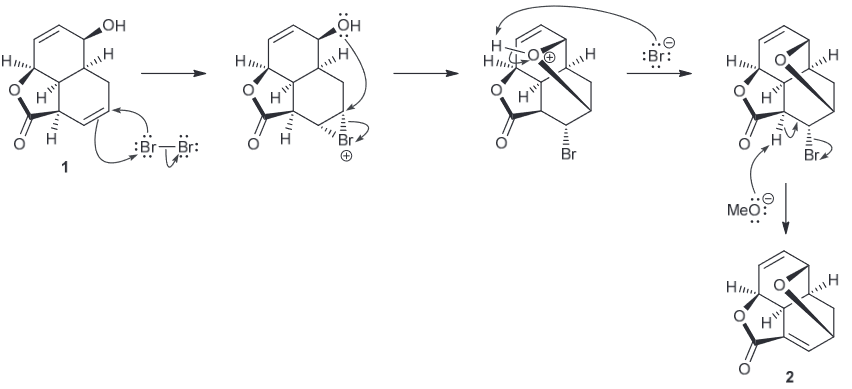
Propose a plausible mechanism for the conversion of 1 to the short lived intermediate 2.